Abstract
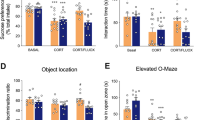
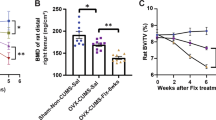
The use of selective serotonin-reuptake inhibitors (SSRIs) has been associated with an increased risk of bone fracture, raising concerns about their increasingly broader usage. This deleterious effect is poorly understood, and thus strategies to avoid this side effect remain elusive. We show here that fluoxetine (Flx), one of the most-prescribed SSRIs, acts on bone remodeling through two distinct mechanisms. Peripherally, Flx has anti-resorptive properties, directly impairing osteoclast differentiation and function through a serotonin-reuptake-independent mechanism that is dependent on intracellular Ca2+ levels and the transcription factor Nfatc1. With time, however, Flx also triggers a brain-serotonin-dependent rise in sympathetic output that increases bone resorption sufficiently to counteract its local anti-resorptive effect, thus leading to a net effect of impaired bone formation and bone loss. Accordingly, neutralizing this second mode of action through co-treatment with the β-blocker propranolol, while leaving the peripheral effect intact, prevents Flx-induced bone loss in mice. Hence, this study identifies a dual mode of action of SSRIs on bone remodeling and suggests a therapeutic strategy to block the deleterious effect on bone homeostasis from their chronic use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wong, D.T., Bymaster, F.P. & Engleman, E.A. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 57, 411–441 (1995).
Fox, M.A. et al. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl.) 195, 147–166 (2007).
Mojtabai, R. & Olfson, M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Aff. (Millwood) 30, 1434–1442 (2011).
Orleans, R.J. et al. FDA approval of paroxetine for menopausal hot flushes. N. Engl. J. Med. 370, 1777–1779 (2014).
Rizzoli, R. et al. Antidepressant medications and osteoporosis. Bone 51, 606–613 (2012).
Haney, E.M., Warden, S.J. & Bliziotes, M.M. Effects of selective serotonin-reuptake inhibitors on bone health in adults: time for recommendations about screening, prevention and management? Bone 46, 13–17 (2010).
Wu, Q., Magnus, J.H., Liu, J., Bencaz, A.F. & Hentz, J.G. Depression and low bone mineral density: a meta-analysis of epidemiologic studies. Osteoporos. Int. 20, 1309–1320 (2009).
Warden, S.J., Nelson, I.R., Fuchs, R.K., Bliziotes, M.M. & Turner, C.H. Serotonin (5-hydroxytryptamine) transporter inhibition causes bone loss in adult mice independently of estrogen deficiency. Menopause 15, 1176–1183 (2008).
Warden, S.J., Robling, A.G., Sanders, M.S., Bliziotes, M.M. & Turner, C.H. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology 146, 685–693 (2005).
Bonnet, N. et al. Various effects of antidepressant drugs on bone microarchitectecture, mechanical properties and bone remodeling. Toxicol. Appl. Pharmacol. 221, 111–118 (2007).
Bliziotes, M., Gunness, M., Eshleman, A. & Wiren, K. The role of dopamine and serotonin in regulating bone mass and strength: studies on dopamine- and serotonin-transporter-null mice. J. Musculoskelet. Neuronal Interact. 2, 291–295 (2002).
Gebara, M.A. et al. Depression, antidepressants and bone health in older adults: a systematic review. J. Am. Geriatr. Soc. 62, 1434–1441 (2014).
Yadav, V.K. et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite and energy expenditure. Cell 138, 976–989 (2009).
Oury, F. et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 24, 2330–2342 (2010).
Seifert, C.F. & Wiltrout, T.R. Calcaneal bone mineral density in young adults prescribed selective serotonin reuptake inhibitors. Clin. Ther. 35, 1412–1417 (2013).
Aydin, H., Mutlu, N. & Akbas, N.B.G. Treatment of a major depression episode suppresses markers of bone turnover in premenopausal women. J. Psychiatr. Res. 45, 1316–1320 (2011).
Misra, M. et al. Use of SSRIs may impact bone density in adolescent and young women with anorexia nervosa. CNS Spectr. 15, 579–586 (2010).
Bolo, N.R. et al. Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology 23, 428–438 (2000).
Boyce, B.F. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 92, 860–867 (2013).
Teitelbaum, S.L. & Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 (2003).
Zou, W. et al. Talin1 and Rap1 are critical for osteoclast function. Mol. Cell. Biol. 33, 830–844 (2013).
Fukunaga, T., Zou, W., Warren, J.T. & Teitelbaum, S.L. Vinculin regulates osteoclast function. J. Biol. Chem. 289, 13554–13564 (2014).
Battaglino, R. et al. Serotonin regulates osteoclast differentiation through its transporter. J. Bone Miner. Res. 19, 1420–1431 (2004).
Hodge, J.M. et al. Selective serotonin-reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol. Psychiatry 74, 32–39 (2013).
Fuller, R.W. & Wong, D.T. Serotonin uptake and serotonin-uptake inhibition. Ann. NY Acad. Sci. 600, 68–78, discussion 79–80 (1990).
Takayanagi, H. Osteoimmunology: shared mechanisms and cross-talk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 (2007).
Chen, W. et al. C/EBP-α regulates osteoclast lineage commitment. Proc. Natl. Acad. Sci. USA 110, 7294–7299 (2013).
Sato, K. et al. Regulation of osteoclast differentiation and function by the CaMK–CREB pathway. Nat. Med. 12, 1410–1416 (2006).
Kuroda, Y., Hisatsune, C., Nakamura, T., Matsuo, K. & Mikoshiba, K. Osteoblasts induce Ca2+-oscillation-independent NFATC1 activation during osteoclastogenesis. Proc. Natl. Acad. Sci. USA 105, 8643–8648 (2008).
Hidaka, H., Asano, M. & Tanaka, T. Activity–structure relationship of calmodulin antagonists, naphthalenesulfonamide derivatives. Mol. Pharmacol. 20, 571–578 (1981).
Best, J.L. et al. Identification of small-molecule antagonists that inhibit an activator–coactivator interaction. Proc. Natl. Acad. Sci. USA 101, 17622–17627 (2004).
Gobin, V. et al. Fluoxetine suppresses calcium signaling in human T lymphocytes through depletion of intracellular calcium stores. Cell Calcium 58, 254–263 (2015).
Okamoto, K. & Takayanagi, H. Regulation of bone by the adaptive immune system in arthritis. Arthritis Res. Ther. 13, 219 (2011).
Takayanagi, H. New developments in osteoimmunology. Nat. Rev. Rheumatol. 8, 684–689 (2012).
Karsenty, G., Kronenberg, H.M. & Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 25, 629–648 (2009).
Karsenty, G. & Ferron, M. The contribution of bone to whole-organism physiology. Nature 481, 314–320 (2012).
Wada, T., Nakashima, T., Hiroshi, N. & Penninger, J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 12, 17–25 (2006).
Ruocco, M.G. et al. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J. Exp. Med. 201, 1677–1687 (2005).
Takeda, S. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002).
Elefteriou, F. et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434, 514–520 (2005).
Gainetdinov, R.R., Premont, R.T., Bohn, L.M., Lefkowitz, R.J. & Caron, M.G. Desensitization of G-protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 27, 107–144 (2004).
Bristow, L.J., O'Connor, D., Watts, R., Duxon, M.S. & Hutson, P.H. Evidence for accelerated de-sensitisation of 5-HT2C receptors following combined treatment with fluoxetine and the 5-HT1A receptor antagonist, WAY 100,635, in the rat. Neuropharmacology 39, 1222–1236 (2000).
Yamauchi, M., Tatebayashi, T., Nagase, K., Kojima, M. & Imanishi, T. Chronic treatment with fluvoxamine desensitizes 5-HT2C-receptor-mediated hypo-locomotion in rats. Pharmacol. Biochem. Behav. 78, 683–689 (2004).
Kennett, G.A. et al. Effect of chronic administration of selective 5-hydroxytryptamine- and noradrenaline-uptake inhibitors on a putative index of 5-HT2C/2B receptor function. Neuropharmacology 33, 1581–1588 (1994).
Chabbi-Achengli, Y. et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc. Natl. Acad. Sci. USA 109, 2567–2572 (2012).
Kim, H.J. et al. Fluoxetine suppresses synaptically induced [Ca2]i spikes and excitotoxicity in cultured rat hippocampal neurons. Brain Res. 1490, 23–34 (2013).
Deák, F. et al. Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology 39, 1029–1036 (2000).
Hahn, S.J. et al. Inhibition by fluoxetine of voltage-activated ion channels in rat PC12 cells. Eur. J. Pharmacol. 367, 113–118 (1999).
Kecskeméti, V. et al. Norfluoxetine and fluoxetine have similar anticonvulsant and Ca2+-channel-blocking potencies. Brain Res. Bull. 67, 126–132 (2005).
Shenoy, A.R. et al. Citalopram suppresses thymocyte cytokine production. J. Neuroimmunol. 262, 46–52 (2013).
Kubera, M., Kenis, G., Bosmans, E., Scharpé, S. & Maes, M. Effects of serotonin, and serotonergic agonists and antagonists, on the production of interferon-γ and interleukin-10. Neuropsychopharmacology 23, 89–98 (2000).
Branco-de-Almeida, L.S. et al. Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. J. Periodontol. 83, 664–671 (2012).
Pellegrino, T.C. & Bayer, B.M. Modulation of immune cell function following fluoxetine administration in rats. Pharmacol. Biochem. Behav. 59, 151–157 (1998).
Schett, G. & Teitelbaum, S.L. Osteoclasts and arthritis. J. Bone Miner. Res. 24, 1142–1146 (2009).
Baharav, E. et al. Immunomodulatory effect of sertraline in a rat model of rheumatoid arthritis. Neuroimmunomodulation 19, 309–318 (2012).
Hochberg, M.C., Wohlreich, M., Gaynor, P., Hanna, S. & Risser, R. Clinically relevant outcomes based on analysis of pooled data from two trials of duloxetine in patients with knee osteoarthritis. J. Rheumatol. 39, 352–358 (2012).
Sacre, S., Medghalchi, M., Gregory, B., Brennan, F. & Williams, R. Fluoxetine and citalopram exhibit potent anti-inflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 62, 683–693 (2010).
Blardi, P. et al. Plasma catecholamine levels after fluoxetine treatment in depressive patients. Neuropsychobiology 51, 72–76 (2005).
Diem, S.J. et al. Effects of escitalopram on markers of bone turnover: a randomized clinical trial. J. Clin. Endocrinol. Metab. 99, E1732–E1737 (2014).
Yadav, V.K. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837 (2008).
Underwood, M.D., Arango, V., Bakalian, M.J., Ruggiero, D.A. & Mann, J.J. Dorsal raphe nucleus serotonergic neurons innervate the rostral ventrolateral medulla in rat. Brain Res. 824, 45–55 (1999).
Ducy, P. & Karsenty, G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15, 1858–1869 (1995).
Angoa-Pérez, M., Kane, M.J., Briggs, D.I., Francescutti, D.M. & Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 82, 50978 (2013).
Nicolas, L.B., Kolb, Y. & Prinssen, E.P.M. A combined marble-burying–locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol. 547, 106–115 (2006).
Acknowledgements
We thank N. Luo for help with histomorphometry, T. Hanna for handling the mouse colony, and G. Karsenty and S. Kousteni for critically reading the manuscript. The US National Institutes of Health grant AG032959 (P.D.) supported this work.
Author information
Authors and Affiliations
Contributions
P.D. conceived the study; M.J.O. performed most of the experiments; S.T.R. performed the μ-CT analysis under X.E.G.'s supervision; P.S. assisted with the calcium signaling analysis under H.M.C.'s supervision; R.P. assisted with the histomorphometry analysis; Y.H. performed the HPLC analysis under J.J.M.'s supervision. M.J.O., H.M.C., J.J.M. and P.D. analyzed and discussed the results; and M.J.O. and P.D. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 7957 kb)
Rights and permissions
About this article
Cite this article
Ortuño, M., Robinson, S., Subramanyam, P. et al. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med 22, 1170–1179 (2016). https://doi.org/10.1038/nm.4166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4166
This article is cited by
-
The Serotonergic System and Bone Metabolism During Pregnancy and Lactation and the Implications of SSRI Use on the Maternal-Offspring Dyad
Journal of Mammary Gland Biology and Neoplasia (2023)
-
Divalent metal cations stimulate skeleton interoception for new bone formation in mouse injury models
Nature Communications (2022)
-
PGE2/EP4 skeleton interoception activity reduces vertebral endplate porosity and spinal pain with low-dose celecoxib
Bone Research (2021)
-
Use of serotonin reuptake inhibitors and risk of subsequent bone loss in a nationwide population-based cohort study
Scientific Reports (2021)
-
The effect of selective serotonin reuptake inhibitors on the human mandible
Oral Radiology (2021)