Abstract
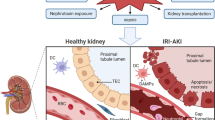
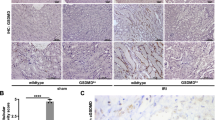
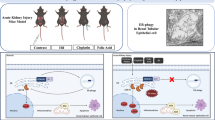
Acute kidney injury (AKI) is associated with prolonged hospitalization and high mortality, and it predisposes individuals to chronic kidney disease. To date, no effective AKI treatments have been established. Here we show that the apoptosis inhibitor of macrophage (AIM) protein on intraluminal debris interacts with kidney injury molecule (KIM)-1 and promotes recovery from AKI. During AKI, the concentration of AIM increases in the urine, and AIM accumulates on necrotic cell debris within the kidney proximal tubules. The AIM present in this cellular debris binds to KIM-1, which is expressed on injured tubular epithelial cells, and enhances the phagocytic removal of the debris by the epithelial cells, thus contributing to kidney tissue repair. When subjected to ischemia-reperfusion (IR)-induced AKI, AIM-deficient mice exhibited abrogated debris clearance and persistent renal inflammation, resulting in higher mortality than wild-type (WT) mice due to progressive renal dysfunction. Treatment of mice with IR-induced AKI using recombinant AIM resulted in the removal of the debris, thereby ameliorating renal pathology. We observed this effect in both AIM-deficient and WT mice, but not in KIM-1–deficient mice. Our findings provide a basis for the development of potentially novel therapies for AKI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Molitoris, B.A. Therapeutic translation in acute kidney injury: the epithelial-endothelial axis. J. Clin. Invest. 124, 2355–2363 (2014).
Susantitaphong, P. et al. World incidence of AKI: a meta-analysis. Clin. J. Am. Soc. Nephrol. 8, 1482–1493 (2013).
Bedford, M., Farmer, C., Levin, A., Ali, T. & Stevens, P. Acute kidney injury and CKD: chicken or egg? Am. J. Kidney Dis. 59, 485–491 (2012).
Amdur, R.L., Chawla, L.S., Amodeo, S., Kimmel, P.L. & Palant, C.E. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 76, 1089–1097 (2009).
Ishani, A. et al. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 20, 223–228 (2009).
Kaushal, G.P. & Shah, S.V. Challenges and advances in the treatment of AKI. J. Am. Soc. Nephrol. 25, 877–883 (2014).
Bonventre, J.V. et al. Kidney Research National Dialogue (KRND). AKI: a path forward. Clin. J. Am. Soc. Nephrol. 8, 1606–1608 (2013).
Jo, S.K., Rosner, M.H. & Okusa, M.D. Pharmacologic treatment of acute kidney injury: why drugs haven't worked, and what is on the horizon. Clin. J. Am. Soc. Nephrol. 2, 356–365 (2007).
Bonventre, J.V. & Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121, 4210–4221 (2011).
Humphreys, B.D. et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 (2008).
Kusaba, T., Lalli, M., Kramann, R., Kobayashi, A. & Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 111, 1527–1532 (2014).
Wan, E. et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ. Res. 113, 1004–1012 (2013).
Juncadella, I.J. et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493, 547–551 (2013).
Henson, P.M., Vandivier, R.W. & Douglas, I.S. Cell death, remodeling and repair in chronic obstructive pulmonary disease? Proc. Am. Thorac. Soc. 3, 713–717 (2006).
Sandahl, M., Hunter, D.M., Strunk, K.E., Earp, H.S. & Cook, R.S. Epithelial cell–directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC Dev. Biol. 10, 122 (2010).
Mochizuki, A. et al. Hepatic stellate cells orchestrate clearance of necrotic cells in a hypoxia-inducible factor–1α–dependent manner by modulating macrophage phenotype in mice. J. Immunol. 192, 3847–3857 (2014).
Ichimura, T. et al. Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 118, 1657–1668 (2008).
Charlton, J.R., Portilla, D. & Okusa, M.D. A basic science view of acute kidney injury biomarkers. Nephrol. Dial. Transplant. 29, 1301–1311 (2014).
Amin, R.P. et al. Identification of putative gene-based markers of renal toxicity. Environ. Health Perspect. 112, 465–479 (2004).
Ichimura, T. et al. Kidney injury molecule–1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is upregulated in renal cells after injury. J. Biol. Chem. 273, 4135–4142 (1998).
Bonventre, J.V. Kidney injury molecule–1: a translational journey. Trans. Am. Clin. Climatol. Assoc. 125, 293–299, discussion 299 (2014).
Xiao, S. et al. Defect in regulatory B cell function and development of systemic autoimmunity in T cell Ig mucin 1 (Tim-1) mucin domain–mutant mice. Proc. Natl. Acad. Sci. USA 109, 12105–12110 (2012).
Yang, L. et al. KIM-1–mediated phagocytosis reduces acute injury to the kidney. J. Clin. Invest. 125, 1620–1636 (2015).
Ismail, O.Z. et al. Kidney injury molecule–1 protects against G-α12 activation and tissue damage in renal ischemia-reperfusion injury. Am. J. Pathol. 185, 1207–1215 (2015).
Miyazaki, T., Hirokami, Y., Matsuhashi, N., Takatsuka, H. & Naito, M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J. Exp. Med. 189, 413–422 (1999).
Yamazaki, T. et al. Circulating AIM as an indicator of liver damage and hepatocellular carcinoma in humans. PLoS One 9, e109123 (2014).
Arai, S. et al. A role for the apoptosis inhibitory factor AIM (Spalpha or Api6) in atherosclerosis development. Cell Metab. 1, 201–213 (2005).
Joseph, S.B. et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119, 299–309 (2004).
Valledor, A.F. et al. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc. Natl. Acad. Sci. USA 101, 17813–17818 (2004).
Arai, S. et al. Obesity-associated autoantibody production requires AIM to retain the immunoglobulin M immune complex on follicular dendritic cells. Cell Reports 3, 1187–1198 (2013).
Sarrias, M.R. et al. A role for human Spalpha as a pattern-recognition receptor. J. Biol. Chem. 280, 35391–35398 (2005).
Kurokawa, J. et al. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 11, 479–492 (2010).
Tissot, J.D. et al. IgM are associated to Spalpha (CD5 antigen-like). Electrophoresis 23, 1203–1206 (2002).
Maehara, N. et al. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Reports 9, 61–74 (2014).
Iwamura, Y. et al. Apoptosis inhibitor of macrophage (AIM) diminishes lipid droplet–coating proteins leading to lipolysis in adipocytes. Biochem. Biophys. Res. Commun. 422, 476–481 (2012).
Arai, S. & Miyazaki, T. Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases. Semin. Immunopathol. 36, 3–12 (2014).
Kurokawa, J. et al. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc. Natl. Acad. Sci. USA 108, 12072–12077 (2011).
Hamada, M. et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat. Commun. 5, 3147 (2014).
Mera, K. et al. Serum levels of apoptosis inhibitor of macrophage are associated with hepatic fibrosis in patients with chronic hepatitis C. BMC Gastroenterol. 14, 27 (2014).
Liaño, F. & Pascual, J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 50, 811–818 (1996).
Mehta, R.L. et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 66, 1613–1621 (2004).
Thadhani, R., Pascual, M. & Bonventre, J.V. Acute renal failure. N. Engl. J. Med. 334, 1448–1460 (1996).
Duffield, J.S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Invest. 124, 2299–2306 (2014).
Linkermann, A., De Zen, F., Weinberg, J., Kunzendorf, U. & Krautwald, S. Programmed necrosis in acute kidney injury. Nephrol. Dial. Transplant. 27, 3412–3419 (2012).
Price, P.M. & Hodeify, R. A possible mechanism of renal cell death after ischemia-reperfusion. Kidney Int. 81, 720–721 (2012).
Linkermann, A. et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia-reperfusion injury. Kidney Int. 81, 751–761 (2012).
Brooks, C., Wei, Q., Cho, S.G. & Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 119, 1275–1285 (2009).
Kim, J., Long, K.E., Tang, K. & Padanilam, B.J. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 82, 193–203 (2012).
Kuchroo, V.K., Umetsu, D.T., DeKruyff, R.H. & Freeman, G.J. The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol. 3, 454–462 (2003).
Angiari, S. & Constantin, G. Regulation of T cell trafficking by the T cell immunoglobulin and mucin domain 1 glycoprotein. Trends Mol. Med. 20, 675–684 (2014).
Lim, A.I., Tang, S.C., Lai, K.N. & Leung, J.C. Kidney injury molecule–1: more than just an injury marker of tubular epithelial cells? J. Cell. Physiol. 228, 917–924 (2013).
Kai, T., Yamazaki, T., Arai, S. & Miyazaki, T. Stabilization and augmentation of circulating AIM in mice by synthesized IgM-Fc. PLoS One 9, e97037 (2014).
Ravichandran, K.S. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity 35, 445–455 (2011).
Yu, W. et al. H2O2 activates G protein–α12 to disrupt the junctional complex and enhance ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 109, 6680–6685 (2012).
Nozaki, Y. et al. Endogenous Tim-1 (Kim-1) promotes T cell responses and cell-mediated injury in experimental crescentic glomerulonephritis. Kidney Int. 81, 844–855 (2012).
Bonventre, J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 14, S55–S61 (2003).
Ferenbach, D.A. & Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney aging and CKD. Nat. Rev. Nephrol. 11, 264–276 (2015).
Chawla, L.S., Eggers, P.W., Star, R.A. & Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66 (2014).
Coca, S.G., Singanamala, S. & Parikh, C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Humphreys, B.D. et al. Chronic epithelial kidney injury molecule–1 expression causes murine kidney fibrosis. J. Clin. Invest. 123, 4023–4035 (2013).
Moore, K.J. et al. A CD36-initiated signaling cascade mediates inflammatory effects of β-amyloid. J. Biol. Chem. 277, 47373–47379 (2002).
Boes, M. et al. Enhanced B-1 cell development, but impaired IgG antibody responses, in mice deficient in secreted IgM. J. Immunol. 160, 4776–4787 (1998).
Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals 8th edn. (The National Academies Press, Washington, D.C., USA, 2011).
Wong, S.H., Barlow, J.L., Nabarro, S., Fallon, P.G. & McKenzie, A.N. Tim-1 is induced on germinal center B cells through B cell–receptor signaling but is not essential for the germinal center response. Immunology 131, 77–88 (2010).
Park, K.M. et al. Inducible nitric oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J. Biol. Chem. 278, 27256–27266 (2003).
Eckardt, K.U. & Kasiske, B.L. Kidney disease: improving global outcomes. Nat. Rev. Nephrol. 5, 650–657 (2009).
Basile, D.P., Anderson, M.D. & Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303–1353 (2012).
Kitamura, S. et al. Transforming growth factor–β1 induces vascular endothelial growth factor expression in murine proximal tubular epithelial cells. Nephron Exp. Nephrol. 95, e79–e86 (2003).
Acknowledgements
We thank M. Nagase, M. Nangaku, T. Fujita, M. Ono, T. Gohda, Y. Sonoda, T. Aruga, K. Kimura, T. Yasuda, Y. Ito and S. Matsuo for helpful advice and discussion; T. Sugaya for mProx24 cells; D. Ikeda and S. Yamashita for Cd36−/− mice; K. Yamamura and N. Takeda for help with animal experiments; A. Nishijima, K. Aoyama and M. Shinohara for general technical assistance; and S. Tomita and Y. Inoue for administrative cooperation. This work was supported by a Core Research for Evolutional Medical Science and Technology grant funded by the Agency for Medical Research and Development (AMED-CREST) (T.M.), a research grant by Onsendo Co., Ltd. (T.M.) and an operating grant from the Canadian Institutes of Health Research (HDK244945) (L.G.).
Author information
Authors and Affiliations
Contributions
S.A. and K.K. performed the majority of experiments—S.A. performed flow cytometry and the in vitro experiments, including the phagocytosis assays, and K.K. did animal experiments and histology. Y.T. and T.Y. contributed to analysis of AKI models; X.Z. and L.G. handled AKI induction in KIM-1–deficient mice; R.T. did histology; R.S., A.M. and Y.Y. contributed to biochemical experiments; T.M. constructed most plasmids; M.M. did kidney cell dissociation; N.M. did immunoblotting of human and mouse sera; S.K. contributed to animal experiments; K.D., A.T., E.N., Y.S. and N.Y. prepared and analyzed human samples; A.N., S.A., T.T. and T.M. designed experiments; and T.M. supervised the whole study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–4 (PDF 2329 kb)
Rights and permissions
About this article
Cite this article
Arai, S., Kitada, K., Yamazaki, T. et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med 22, 183–193 (2016). https://doi.org/10.1038/nm.4012
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4012