Abstract
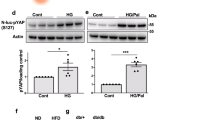
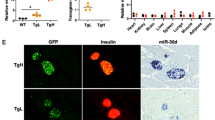
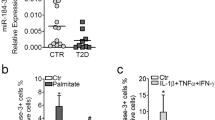
Pancreatic beta cell death is a hallmark of type 1 (T1D) and type 2 (T2D) diabetes, but the molecular mechanisms underlying this aspect of diabetic pathology are poorly understood. Here we report that expression of the microRNA (miR)-200 family is strongly induced in islets of diabetic mice and that beta cell–specific overexpression of miR-200 in mice is sufficient to induce beta cell apoptosis and lethal T2D. Conversely, mir-200 ablation in mice reduces beta cell apoptosis and ameliorates T2D. We show that miR-200 negatively regulates a conserved anti-apoptotic and stress-resistance network that includes the essential beta cell chaperone Dnajc3 (also known as p58IPK) and the caspase inhibitor Xiap. We also observed that mir-200 dosage positively controls activation of the tumor suppressor Trp53 and thereby creates a pro-apoptotic gene-expression signature found in islets of diabetic mice. Consequently, miR-200–induced T2D is suppressed by interfering with the signaling of Trp53 and Bax, a proapoptotic member of the B cell lymphoma 2 protein family. Our results reveal a crucial role for the miR-200 family in beta cell survival and the pathophysiology of diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Butler, A.E. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003).
Song, B., Scheuner, D., Ron, D., Pennathur, S. & Kaufman, R.J. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118, 3378–3389 (2008).
Barlow, A.D., Nicholson, M.L. & Herbert, T.P. Evidence for rapamycin toxicity in pancreatic beta-cells and a review of the underlying molecular mechanisms. Diabetes 62, 2674–2682 (2013).
Robertson, R.P., Harmon, J., Tran, P.O. & Poitout, V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53 (suppl. 1), S119–S124 (2004).
Volchuk, A. & Ron, D. The endoplasmic reticulum stress response in the pancreatic beta-cell. Diabetes Obes. Metab. 12 (suppl. 2), 48–57 (2010).
Halban, P.A. et al. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37, 1751–1758 (2014).
Friedman, R.C., Farh, K.K., Burge, C.B. & Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 (2009).
Bartel, D.P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Poy, M.N. et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432, 226–230 (2004).
Latreille, M. et al. MicroRNA-7a regulates pancreatic beta cell function. J. Clin. Invest. 124, 2722–2735 (2014).
Kameswaran, V. et al. Epigenetic regulation of the DLK1–MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab. 19, 135–145 (2014).
Xu, G., Chen, J., Jing, G. & Shalev, A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 19, 1141–1146 (2013).
Kornfeld, J.W. et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494, 111–115 (2013).
Vidigal, J.A. & Ventura, A. The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol. 25, 137–147 (2015).
Trajkovski, M., Ahmed, K., Esau, C.C. & Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 14, 1330–1335 (2012).
Park, S.M., Gaur, A.B., Lengyel, E. & Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 22, 894–907 (2008).
Kim, Y.K. et al. TALEN-based knockout library for human microRNAs. Nat. Struct. Mol. Biol. 20, 1458–1464 (2013).
Burk, U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9, 582–589 (2008).
Song, S.J. et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 154, 311–324 (2013).
Korpal, M. et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 17, 1101–1108 (2011).
Hasuwa, H., Ueda, J., Ikawa, M. & Okabe, M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 341, 71–73 (2013).
Klein, D. et al. MicroRNA expression in alpha and beta cells of human pancreatic islets. PLoS ONE 8, e55064 (2013).
Filios, S.R. et al. MicroRNA-200 is induced by thioredoxin-interacting protein and regulates zeb1 protein signaling and beta cell apoptosis. J. Biol. Chem. 289, 36275–36283 (2014).
Pullen, T.J., da Silva Xavier, G., Kelsey, G. & Rutter, G.A. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol. Cell. Biol. 31, 3182–3194 (2011).
Puff, R. et al. Reduced proliferation and a high apoptotic frequency of pancreatic beta cells contribute to genetically-determined diabetes susceptibility of db/db BKS mice. Horm. Metab. Res. 43, 306–311 (2011).
Ardestani, A. et al. MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat. Med. 20, 385–397 (2014).
Talchai, C., Xuan, S., Lin, H.V., Sussel, L. & Accili, D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150, 1223–1234 (2012).
Mandelbaum, A.D. et al. Dysregulation of Dicer1 in beta cells impairs islet architecture and glucose metabolism. Exp. Diabetes Res. 2012, 470302 (2012).
Leung, A.K. & Sharp, P.A. microRNAs: a safeguard against turmoil? Cell 130, 581–585 (2007).
Tornovsky-Babeay, S. et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in beta cells. Cell Metab. 19, 109–121 (2014).
Tonne, J.M. et al. Global gene expression profiling of pancreatic islets in mice during streptozotocin-induced beta-cell damage and pancreatic Glp-1 gene therapy. Dis. Model. Mech. 6, 1236–1245 (2013).
Eichhorn, S.W. et al. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol. Cell 56, 104–115 (2014).
Ladiges, W.C. et al. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes 54, 1074–1081 (2005).
Zeggini, E. et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 40, 638–645 (2008).
Marselli, L. et al. Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS ONE 5, e11499 (2010).
Harada, H., Andersen, J.S., Mann, M., Terada, N. & Korsmeyer, S.J. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA 98, 9666–9670 (2001).
Danial, N.N. et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat. Med. 14, 144–153 (2008).
Emamaullee, J.A. et al. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes 54, 2541–2548 (2005).
Plesner, A., Liston, P., Tan, R., Korneluk, R.G. & Verchere, C.B. The X-linked inhibitor of apoptosis protein enhances survival of murine islet allografts. Diabetes 54, 2533–2540 (2005).
Harlin, H., Reffey, S.B., Duckett, C.S., Lindsten, T. & Thompson, C.B. Characterization of XIAP-deficient mice. Mol. Cell. Biol. 21, 3604–3608 (2001).
Yan, W. et al. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl. Acad. Sci. USA 99, 15920–15925 (2002).
Shang, X. et al. Dual-specificity phosphatase 26 is a novel p53 phosphatase and inhibits p53 tumor suppressor functions in human neuroblastoma. Oncogene 29, 4938–4946 (2010).
Armata, H.L., Garlick, D.S. & Sluss, H.K. The ataxia telangiectasia-mutated target site Ser18 is required for p53-mediated tumor suppression. Cancer Res. 67, 11696–11703 (2007).
Couch, F.J. et al. Association of mitotic regulation pathway polymorphisms with pancreatic cancer risk and outcome. Cancer Epidemiol. Biomarkers Prev. 19, 251–257 (2010).
Cunha, D.A. et al. Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human beta-cell apoptosis. Diabetes 61, 2763–2775 (2012).
McKenzie, M.D. et al. Glucose induces pancreatic islet cell apoptosis that requires the BH3-only proteins Bim and Puma and multi-BH domain protein Bax. Diabetes 59, 644–652 (2010).
Hartman, M.G. et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol. Cell. Biol. 24, 5721–5732 (2004).
Kawase, T. et al. PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt. Cell 136, 535–550 (2009).
Hashimoto, N. et al. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat. Genet. 38, 589–593 (2006).
Hanahan, D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 315, 115–122 (1985).
Chipuk, J.E. et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, 1010–1014 (2004).
Krützfeldt, J. et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature 438, 685–689 (2005).
Obad, S. et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet. 43, 371–378 (2011).
Chang, C.J. et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 13, 317–323 (2011).
Jiang, H.Y. et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol. Cell. Biol. 24, 1365–1377 (2004).
Zmuda, E.J. et al. Deficiency of Atf3, an adaptive-response gene, protects islets and ameliorates inflammation in a syngeneic mouse transplantation model. Diabetologia 53, 1438–1450 (2010).
Thomas, G. et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat. Genet. 40, 310–315 (2008).
Ohki, R. et al. PHLDA3 is a novel tumor suppressor of pancreatic neuroendocrine tumors. Proc. Natl. Acad. Sci. USA 111, E2404–E2413 (2014).
Ranger, A.M. et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 100, 9324–9329 (2003).
Olive, K.P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004).
Li, B. & Dewey, C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Denzler, R., Agarwal, V., Stefano, J., Bartel, D.P. & Stoffel, M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell 54, 766–776 (2014).
Friedman, R.C., Farh, K.K.-H., Burge, C.B. & Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 (2009).
Vassilev, L.T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Acknowledgements
We wish to thank B. Kaps and R. Kubsch for excellent technical and animal husbandry assistance. We thank the Functional Genomics Center Zurich and the Light Microscopy and Screening Center Zurich for support, and N. Danial (Dana Farber Cancer Institute, Boston, USA), D. Hanahan (Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland) and P. Herrera (University of Geneva, Switzerland) for sharing Bad−/−, Rip-Tag2 and Rip-Cre mice, respectively. This work was supported by European Molecular Biology Organization Long-Term Fellowships (to B.-F.B. and K.A.), the Austrian Genome Research Programme GEN-AU II and III (Austromouse) (to T.R.) and the European Genomic Institute for Diabetes (ANR-10-LABX-46 to F.P.) and in part sponsored by a Juvenile Diabetes Research Foundation (JDRF) Scholar award, European Research Council grant 'Metabolomirs', the Starr Foundation International and the Swiss National Science Foundation, and the National Center of Competence in Research on RNA Biology and Disease (all to M.S.). Human islets for research were provided thanks to the European Consortium for Islet Transplantation funded by the JDRF (31-2012-783 to F.P.).
Author information
Authors and Affiliations
Contributions
B.-F.B. and K.A. performed most of the experiments, analyzed and interpreted data and wrote the manuscript; K.A. generated conditional miR-200aflox/flox mice; M.S. generated Rip141/200c and mir-141/200c−/− animals and performed the initial characterizations; M.L. generated miR expression data in islets of obese mice; R.D. performed the bioinformatics analysis; N.K., F.v.M., F.N.V. and K.H. helped with some of the in vivo experiments and immunohistochemistry; D.B., J.K.-C. and F.P. obtained human islets and performed quality controls; T.R. performed pronuclei and blastocyst injections; M.S. analyzed and interpreted data, supervised the project and wrote the manuscript.
Ethics declarations
Competing interests
M.S. is a member of the scientific advisory boards of Regulus Therapeutics and Alnylam Pharmaceuticals
Supplementary information
Supplementary Text and Figures
Supplementary figures 1–10 & Supplementary Tables 1–2 (PDF 9996 kb)
Rights and permissions
About this article
Cite this article
Belgardt, BF., Ahmed, K., Spranger, M. et al. The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat Med 21, 619–627 (2015). https://doi.org/10.1038/nm.3862
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3862
This article is cited by
-
Are Vascular Endothelium and Angiogenesis Effective MicroRNA Biomarkers Associated with the Prediction of Early-Onset Preeclampsia (EOPE) and Adverse Perinatal Outcomes?
Reproductive Sciences (2024)
-
miRNA expression in PCOS: unveiling a paradigm shift toward biomarker discovery
Archives of Gynecology and Obstetrics (2024)
-
Circulating miRNA expression in long-standing type 1 diabetes mellitus
Scientific Reports (2023)
-
p53 Inhibition in Pancreatic Progenitors Enhances the Differentiation of Human Pluripotent Stem Cells into Pancreatic β-Cells
Stem Cell Reviews and Reports (2023)
-
Sphingolipid subtypes differentially control proinsulin processing and systemic glucose homeostasis
Nature Cell Biology (2023)