Abstract
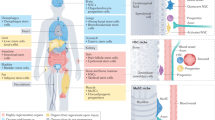
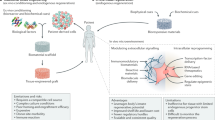
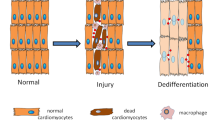
Chronic diseases confer tissue and organ damage that reduce quality of life and are largely refractory to therapy. Although stem cells hold promise for treating degenerative diseases by 'seeding' injured tissues, the regenerative capacity of stem cells is influenced by regulatory networks orchestrated by local immune responses to tissue damage, with macrophages being a central component of the injury response and coordinator of tissue repair. Recent research has turned to how cellular and signaling components of the local stromal microenvironment (the 'soil' to the stem cells' seed), such as local inflammatory reactions, contribute to successful tissue regeneration. This Review discusses the basic principles of tissue regeneration and the central role locally acting components may play in the process. Application of seed-and-soil concepts to regenerative medicine strengthens prospects for developing cell-based therapies or for promotion of endogenous repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Debbie Maizels / Nature Publishing Group

Debbie Maizels / Nature Publishing Group

Debbie Maizels / Nature Publishing Group

Debbie Maizels / Nature Publishing Group
Similar content being viewed by others
References
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Niethammer, P., Grabher, C., Look, A.T. & Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 (2009).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Pase, L. et al. Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Curr. Biol. 22, 1818–1824 (2012).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Skaper, S.D., Facci, L. & Giusti, P. Mast cells, glia and neuroinflammation: partners in crime? Immunology 141, 314–327 (2014).
Wulff, B.C. & Wilgus, T.A. Mast cell activity in the healing wound: more than meets the eye? Exp. Dermatol. 22, 507–510 (2013).
Porta, C. et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl. Acad. Sci. USA 106, 14978–14983 (2009).
Martinez, F.O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Wynn, T.A., Chawla, A. & Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Mosser, D.M. & Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Davies, L.C., Jenkins, S.J., Allen, J.E. & Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
Jenkins, S.J. et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J. Exp. Med. 210, 2477–2491 (2013).
Jenkins, S.J. et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011).
Li, L., Yan, B., Shi, Y.Q., Zhang, W.Q. & Wen, Z.L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 287, 25353–25360 (2012).
Hay, E.D. & Fischman, D.A. Origin of the blastema in regenerating limbs of the newt Triturus viridescens. An autoradiographic study using tritiated thymidine to follow cell proliferation and migration. Dev. Biol. 3, 26–59 (1961).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Chen, C.W. et al. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 20, 429–434 (2009).
Dulauroy, S., Di Carlo, S.E., Langa, F., Eberl, G. & Peduto, L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18, 1262–1270 (2012).
Lin, S.L., Kisseleva, T., Brenner, D.A. & Duffield, J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Henderson, N.C. et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Göritz, C. et al. A pericyte origin of spinal cord scar tissue. Science 333, 238–242 (2011).
Issa, R. et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCL4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 17, 47–49 (2003).
Kallis, Y.N. et al. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut 60, 525–533 (2011).
Pellicoro, A., Ramachandran, P., Iredale, J.P. & Fallowfield, J.A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
Stark, K. et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat. Immunol. 14, 41–51 (2013).
Dellavalle, A. et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2, 499 (2011).
Cappellari, O. et al. Dll4 and PDGF-BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev. Cell 24, 586–599 (2013).
Katare, R. et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 109, 894–906 (2011).
Corselli, M., Chen, C.W., Crisan, M., Lazzari, L. & Peault, B. Perivascular ancestors of adult multipotent stem cells. Arterioscler. Thromb. Vasc. Biol. 30, 1104–1109 (2010).
Wing, K. & Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11, 7–13 (2010).
Josefowicz, S.Z., Lu, L.F. & Rudensky, A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Burzyn, D., Benoist, C. & Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 14, 1007–1013 (2013).
Tang, T.T. et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 107, 232 (2012).
Michalopoulos, G.K. & DeFrances, M.C. Liver regeneration. Science 276, 60–66 (1997).
Bird, T.G., Lorenzini, S. & Forbes, S.J. Activation of stem cells in hepatic diseases. Cell Tissue Res. 331, 283–300 (2008).
Boulton, R.A., Alison, M.R., Golding, M., Selden, C. & Hodgson, H.J. Augmentation of the early phase of liver regeneration after 70% partial hepatectomy in rats following selective Kupffer cell depletion. J. Hepatol. 29, 271–280 (1998).
Boulton, R. et al. Nonparenchymal cells from regenerating rat liver generate interleukin-1α and -1β: a mechanism of negative regulation of hepatocyte proliferation. Hepatology 26, 49–58 (1997).
Meijer, C. et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver 20, 66–77 (2000).
Goh, Y.P. et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA 110, 9914–9919 (2013).
Higgins, G.M. & Anderson, R.M. Experimental pathology of the liver: restoration of the liver of the white rat following partial removal. Arch. Pathol. (Chic.) 12, 186–202 (1931).
Yamanaka, N. et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 18, 79–85 (1993).
Ding, B.S. et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468, 310–315 (2010).
Hu, J. et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science 343, 416–419 (2014).
Ding, B.S. et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505, 97–102 (2014).
Passino, M.A., Adams, R.A., Sikorski, S.L. & Akassoglou, K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science 315, 1853–1856 (2007).
Ochoa, B. et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology 51, 1712–1723 (2010).
Issa, R. et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 17, 47–49 (2003).
Boulter, L. et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579 (2012).
Tsuchiya, A. et al. PolySia-NCAM modulates the formation of ductular reactions in liver injury. Hepatology doi:10.1002/hep.27099 (28 February 2014).
Williams, M.J., Clouston, A.D. & Forbes, S.J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 146, 349–356 (2014).
Iredale, J.P., Murphy, G., Hembry, R.M., Friedman, S.L. & Arthur, M.J. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J. Clin. Invest. 90, 282–287 (1992).
Iredale, J.P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 (1998).
Fallowfield, J.A. et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 178, 5288–5295 (2007).
Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. USA 109, 9448–9453 (2012).
Duffield, J.S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Gibbons, M.A. et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 184, 569–581 (2011).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 109, E3186–E3195 (2012).
Overturf, K., al-Dhalimy, M., Ou, C.N., Finegold, M. & Grompe, M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am. J. Pathol. 151, 1273–1280 (1997).
Bird, T.G. et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc. Natl. Acad. Sci. USA 110, 6542–6547 (2013).
Espanol-Suner, R. et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143, 1564–1575.e7 (2012).
He, J., Lu, H., Zou, Q. & Luo, L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146, 789–800.e8 (2014).
Choi, T.Y., Ninov, N., Stainier, D.Y. & Shin, D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 146, 776–788 (2014).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Yanger, K. et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 27, 719–724 (2013).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Barker, N. et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Takeda, N. et al. Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424 (2011).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Jung, P. et al. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 17, 1225–1227 (2011).
Sala, F.G. et al. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng. Part A 17, 1841–1850 (2011).
MacDonald, T.T., Monteleone, I., Fantini, M.C. & Monteleone, G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology 140, 1768–1775 (2011).
Pull, S.L., Doherty, J.M., Mills, J.C., Gordon, J.I. & Stappenbeck, T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 102, 99–104 (2005).
Lu, N. et al. Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. J. Immunol. 192, 1013–1023 (2014).
Seno, H. et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl. Acad. Sci. USA 106, 256–261 (2009).
Egea, L. et al. GM-CSF produced by nonhematopoietic cells is required for early epithelial cell proliferation and repair of injured colonic mucosa. J. Immunol. 190, 1702–1713 (2013).
Willenborg, S. & Eming, S.A. Macrophages—sensors and effectors coordinating skin damage and repair. J. Dtsch. Dermatol. Ges. 12, 214–221 (2014).
Lucas, T. et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 184, 3964–3977 (2010).
Russell, S.E. & Walsh, P.T. Sterile inflammation—do innate lymphoid cell subsets play a role? Front. Immunol. 3, 246 (2012).
Rabelink, T.J. & Little, M.H. Stromal cells in tissue homeostasis: balancing regeneration and fibrosis. Nat. Rev. Nephrol. 9, 747–753 (2013).
Anders, H.J. Immune system modulation of kidney regeneration-mechanisms and implications. Nat. Rev. Nephrol. 10, 347–358 (2014).
Romagnani, P., Lasagni, L. & Remuzzi, G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat. Rev. Nephrol. 9, 137–146 (2013).
McCampbell, K.K. & Wingert, R.A. New tides: using zebrafish to study renal regeneration. Transl. Res. 163, 109–122 (2014).
LeBleu, V.S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Wang, Y. et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 72, 290–299 (2007).
Alikhan, M.A. et al. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am. J. Pathol. 179, 1243–1256 (2011).
Carosio, S., Berardinelli, M.G., Aucello, M. & Musaro, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 10, 35–42 (2011).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Tidball, J.G. & Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173–R1187 (2010).
Saclier, M. et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31, 384–396 (2013).
Massimino, M.L. et al. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem. Biophys. Res. Commun. 235, 754–759 (1997).
Villalta, S.A., Nguyen, H.X., Deng, B., Gotoh, T. & Tidball, J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 18, 482–496 (2009).
Lu, H. et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25, 358–369 (2011).
Sica, A., Schioppa, T., Mantovani, A. & Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur. J. Cancer 42, 717–727 (2006).
Ochoa, O. et al. Delayed angiogenesis and VEGF production in Ccr2−/− mice during impaired skeletal muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R651–R661 (2007).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Ruffell, D. et al. A CREB-C/EBPb cascade induces M2 macrophage–specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 106, 17475–17480 (2009).
Villalta, S.A. et al. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum. Mol. Genet. 20, 790–805 (2011).
Tidball, J.G. & Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173–R1187 (2010).
Swirski, F.K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Taghavie-Moghadam, P.L., Butcher, M.J. & Galkina, E.V. The dynamic lives of macrophage and dendritic cell subsets in atherosclerosis. Ann. NY Acad. Sci. 1319, 19–37 (2014).
Pinto, A.R. et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS ONE 7, e36814 (2012).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Gow, D.J., Sester, D.P. & Hume, D.A. CSF-1, IGF-1, and the control of postnatal growth and development. J. Leukoc. Biol. 88, 475–481 (2010).
Santini, M.P. et al. Enhancing repair of the mammalian heart. Circ. Res. 100, 1732–1740 (2007).
Iekushi, K., Seeger, F., Assmus, B., Zeiher, A.M. & Dimmeler, S. Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. Circulation 125, 1765–1773, S1–S7 (2012).
Burchfield, J.S. et al. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ. Res. 103, 203–211 (2008).
Zangi, L. et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 31, 898–907 (2013).
Smart, N. et al. Myocardial regeneration: expanding the repertoire of thymosin beta4 in the ischemic heart. Ann. NY Acad. Sci. 1269, 92–101 (2012).
Kikuchi, K. & Poss, K.D. Cardiac regenerative capacity and mechanisms. Annu. Rev. Cell Dev. Biol. 28, 719–741 (2012).
Porrello, E.R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Porrello, E.R. et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 110, 187–192 (2013).
Haubner, B.J. et al. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 4, 966–977 (2012).
Leor, J. et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation 114, I94–I100 (2006).
Cho, D.I. et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 46, e70 (2014).
Franklin, R.J. & Ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Czopka, T., Ffrench-Constant, C. & Lyons, D.A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013).
Gibson, E.M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
London, A., Cohen, M. & Schwartz, M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front. Cell. Neurosci. 7, 34 (2013).
Shechter, R. et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38, 555–569 (2013).
Shechter, R., Raposo, C., London, A., Sagi, I. & Schwartz, M. The glial scar-monocyte interplay: a pivotal resolution phase in spinal cord repair. PLoS ONE 6, e27969 (2011).
Miron, V.E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013).
Schwartz, M. & Raposo, C. Protective Autoimmunity: A unifying model for the immune network involved in CNS repair. Neuroscientist doi:10.1177/1073858413516799 (6 January 2014).
Butovsky, O., Talpalar, A.E., Ben-Yaakov, K. & Schwartz, M. Activation of microglia by aggregated β-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-γ and IL-4 render them protective. Mol. Cell. Neurosci. 29, 381–393 (2005).
Ziv, Y., Avidan, H., Pluchino, S., Martino, G. & Schwartz, M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc. Natl. Acad. Sci. USA 103, 13174–13179 (2006).
Psaltis, P.J., Simari, R.D. & Rodriguez-Porcel, M. Emerging roles for integrated imaging modalities in cardiovascular cell-based therapeutics: a clinical perspective. Eur. J. Nucl. Med. Mol. Imaging 39, 165–181 (2012).
Lammers, G. et al. An overview of methods for the in vivo evaluation of tissue-engineered skin constructs. Tissue Eng. Part B Rev. 17, 33–55 (2011).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
Hay, D.C. et al. Unbiased screening of polymer libraries to define novel substrates for functional hepatocytes with inducible drug metabolism. Stem Cell Res. 6, 92–102 (2011).
Mendelson, A. & Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 20, 833–846 (2014).
Behfar, A., Crespo-Diaz, R., Terzic, A. & Gersh, B.J. Cell therapy for cardiac repair—lessons from clinical trials. Nat. Rev. Cardiol. 11, 232–246 (2014).
Cheng, K., Wu, F. & Cao, F. Intramyocardial autologous cell engraftment in patients with ischaemic heart failure: a meta-analysis of randomised controlled trials. Heart Lung Circ. 22, 887–894 (2013).
Rosado-de-Castro, P.H., Pimentel-Coelho, P.M., da Fonseca, L.M., de Freitas, G.R. & Mendez-Otero, R. The rise of cell therapy trials for stroke: review of published and registered studies. Stem Cells Dev. 22, 2095–2111 (2013).
Ellis, E.L. & Mann, D.A. Clinical evidence for the regression of liver fibrosis. J. Hepatol. 56, 1171–1180 (2012).
Soltys, K.A. et al. Barriers to the successful treatment of liver disease by hepatocyte transplantation. J. Hepatol. 53, 769–774 (2010).
Thomas, J.A. et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology 53, 2003–2015 (2011).
Nakamura, T. et al. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology 133, 91–107.e1 (2007).
Wang, L., Wang, X., Xie, G., Hill, C.K. & DeLeve, L.D. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J. Clin. Invest. 122, 1567–1573 (2012).
Turner, M. et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 13, 382–384 (2013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Forbes, S., Rosenthal, N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 20, 857–869 (2014). https://doi.org/10.1038/nm.3653
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3653
This article is cited by
-
Fibroblast and myofibroblast activation in normal tissue repair and fibrosis
Nature Reviews Molecular Cell Biology (2024)
-
Nanosilicate-functionalized nanofibrous membrane facilitated periodontal regeneration potential by harnessing periodontal ligament cell-mediated osteogenesis and immunomodulation
Journal of Nanobiotechnology (2023)
-
A system for bioelectronic delivery of treatment directed toward wound healing
Scientific Reports (2023)
-
Cellular and Molecular Mechanisms Underly the Combined Treatment of Fasudil and Bone Marrow Derived-Neuronal Stem Cells in a Parkinson’s Disease Mouse Model
Molecular Neurobiology (2023)
-
Harnessing Biomaterials for Immunomodulatory-Driven Tissue Engineering
Regenerative Engineering and Translational Medicine (2023)