Abstract
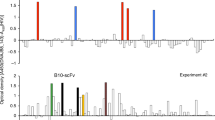
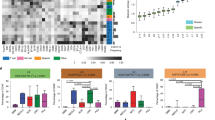
Immune evasion is an emerging hallmark of cancer progression. However, functional studies to understand the role of myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment are limited by the lack of available specific cell surface markers. We adapted a competitive peptide phage display platform to identify candidate peptides binding MDSCs specifically and generated peptide-Fc fusion proteins (peptibodies). In multiple tumor models, intravenous peptibody injection completely depleted blood, splenic and intratumoral MDSCs in tumor-bearing mice without affecting proinflammatory immune cell types, such as dendritic cells. Whereas control Gr-1–specific antibody primarily depleted granulocytic MDSCs, peptibodies depleted both granulocytic and monocytic MDSC subsets. Peptibody treatment was associated with inhibition of tumor growth in vivo, which was superior to that achieved with Gr-1–specific antibody. Immunoprecipitation of MDSC membrane proteins identified S100 family proteins as candidate targets. Our strategy may be useful to identify new diagnostic and therapeutic surface targets on rare cell subtypes, including human MDSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cheever, M.A. & Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 17, 3520–3526 (2011).
Schwartzentruber, D.J. et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 364, 2119–2127 (2011).
Schuster, S.J. et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J. Clin. Oncol. 29, 2787–2794 (2011).
Marigo, I., Dolcetti, L., Serafini, P., Zanovello, P. & Bronte, V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 222, 162–179 (2008).
Gabrilovich, D.I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Peranzoni, E. et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22, 238–244 (2010).
Bronte, V. & Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Rodriguez, P.C. et al. Regulation of T cell receptor CD3ζ chain expression by l-arginine. J. Biol. Chem. 277, 21123–21129 (2002).
Ochoa, A.C., Zea, A.H., Hernandez, C. & Rodriguez, P.C. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 13, 721s–726s (2007).
Ugel, S. et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr. Opin. Pharmacol. 9, 470–481 (2009).
Vogl, T. et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 104, 4260–4268 (2004).
Cheng, P. et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249 (2008).
Sinha, P. et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 181, 4666–4675 (2008).
Ichikawa, M., Williams, R., Wang, L., Vogl, T. & Srikrishna, G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol. Cancer Res. 9, 133–148 (2011).
Källberg, E. et al. S100A9 interaction with TLR4 promotes tumor growth. PLoS ONE 7, e34207 (2012).
Zhao, F. et al. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology 136, 176–183 (2012).
Acknowledgements
This work was supported by a Developmental Research Program award to H.Q. from the US National Cancer Institute Specialized Programs of Research Excellence in Lymphoma (P50 CA136411). We thank D. Hawke for performing proteomic sequencing and analyzing results, D. Kusewitt (M.D. Anderson Cancer Center) for providing S100A9-deficient mice and D. Gwak for editing assistance.
Author information
Authors and Affiliations
Contributions
H.Q. designed the project and experiments, analyzed data and wrote the manuscript. B.L. and I.S. performed most of the experiments. G.W., S.S.R., S.C.C., J.Q., Y.H., R.N. and K.C.D. assisted with mouse studies, flow cytometry experiments and cell sorting. J.R., Q.Y. and W.W.O. provided S100A9-knockout mice, the EL4 tumor model and Gr-1–specific mAb, respectively, and also reviewed the manuscript. L.W.K. supervised the project, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1 and 2 (PDF 1837 kb)
Rights and permissions
About this article
Cite this article
Qin, H., Lerman, B., Sakamaki, I. et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 20, 676–681 (2014). https://doi.org/10.1038/nm.3560
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3560
This article is cited by
-
Immune modulation in malignant pleural effusion: from microenvironment to therapeutic implications
Cancer Cell International (2024)
-
Single-cell transcriptome profiling of sepsis identifies HLA-DRlowS100Ahigh monocytes with immunosuppressive function
Military Medical Research (2023)
-
Tumor-associated myeloid cells in cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
Exploiting innate immunity for cancer immunotherapy
Molecular Cancer (2023)
-
Emerging biomaterials for tumor immunotherapy
Biomaterials Research (2023)