Abstract
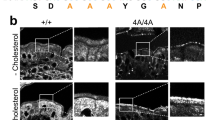
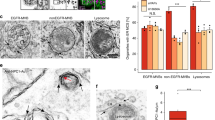
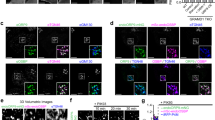
Hypercholesterolemia, typically due to excessive cholesterol uptake, is a major risk factor for cardiovascular disease, which is responsible for ∼50% of all deaths in developed societies. Although it has been shown that intestinal cholesterol absorption is mediated by vesicular endocytosis of the Niemann-Pick C1–like 1 (NPC1L1) protein1,2, the mechanism of sterol-stimulated NPC1L1 internalization is still mysterious. Here, we identified an endocytic peptide signal, YVNXXF (where X stands for any amino acid), in the cytoplasmic C-terminal tail of NPC1L1. Cholesterol binding on the N-terminal domain of NPC1L1 released the YVNXXF-containing region of NPC1L1 from association with the plasma membrane and enabled Numb binding. We also found that Numb, a clathrin adaptor, specifically recognized this motif and recruited clathrin for internalization. Disrupting the NPC1L1-Numb interaction decreased cholesterol uptake. Ablation of Numb in mouse intestine significantly reduced dietary cholesterol absorption and plasma cholesterol level. Together, these data show that Numb is a pivotal protein for intestinal cholesterol absorption and may provide a therapeutic target for hypercholesterolemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Altmann, S.W. et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Ge, L. et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7, 508–519 (2008).
Temel, R.E. et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Invest. 117, 1968–1978 (2007).
Davis, H.R. Jr. et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279, 33586–33592 (2004).
Davis, H.R. Jr. & Altmann, S.W. Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. Biochim. Biophys. Acta 1791, 679–683 (2009).
Wang, L.J. & Song, B.L. Niemann-Pick C1-Like 1 and cholesterol uptake. Biochim. Biophys. Acta 1821, 964–972 (2012).
Chang, T.Y. & Chang, C. Ezetimibe blocks internalization of the NPC1L1/cholesterol complex. Cell Metab. 7, 469–471 (2008).
Zhang, J.H. et al. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. J. Biol. Chem. 286, 25088–25097 (2011).
Kwon, H.J., Palnitkar, M. & Deisenhofer, J. The structure of the NPC1L1 N-terminal domain in a closed conformation. PLoS ONE 6, e18722 (2011).
Ge, L. et al. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl. Acad. Sci. USA 108, 551–556 (2011).
Weinglass, A.B. et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc. Natl. Acad. Sci. USA 105, 11140–11145 (2008).
Chang, T.Y., Chang, C.C., Ohgami, N. & Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22, 129–157 (2006).
Davies, J.P., Scott, C., Oishi, K., Liapis, A. & Ioannou, Y.A. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 280, 12710–12720 (2005).
Wang, L.J. et al. Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J. Biol. Chem. 286, 7397–7408 (2011).
Gulino, A., Di Marcotullio, L. & Screpanti, I. The multiple functions of Numb. Exp. Cell Res. 316, 900–906 (2010).
Roncarati, R. et al. The γ-secretase–generated intracellular domain of β-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc. Natl. Acad. Sci. USA 99, 7102–7107 (2002).
Dho, S.E. et al. The mammalian numb phosphotyrosine-binding domain. Characterization of binding specificity and identification of a novel PDZ domain-containing numb binding protein, LNX. J. Biol. Chem. 273, 9179–9187 (1998).
Nishimura, T. & Kaibuchi, K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell 13, 15–28 (2007).
Tong, X. et al. Numb independently antagonizes Sanpodo membrane targeting and Notch signaling in Drosophila sensory organ precursor cells. Mol. Biol. Cell 21, 802–810 (2010).
Aivazian, D. & Stern, L.J. Phosphorylation of T cell receptor ζ is regulated by a lipid dependent folding transition. Nat. Struct. Biol. 7, 1023–1026 (2000).
Wang, J. et al. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J. Lipid Res. 50, 1653–1662 (2009).
Motamed, M. et al. Identification of luminal Loop 1 of Scap protein as the sterol sensor that maintains cholesterol homeostasis. J. Biol. Chem. 286, 18002–18012 (2011).
Kwon, H.J. et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137, 1213–1224 (2009).
Klein, A.L., Zilian, O., Suter, U. & Taylor, V. Murine numb regulates granule cell maturation in the cerebellum. Dev. Biol. 266, 161–177 (2004).
Li, H.S. et al. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron 40, 1105–1118 (2003).
Colaluca, I.N. et al. NUMB controls p53 tumour suppressor activity. Nature 451, 76–80 (2008).
Nie, J. et al. LNX functions as a RING type E3 ubiquitin ligase that targets the cell fate determinant Numb for ubiquitin-dependent degradation. EMBO J. 21, 93–102 (2002).
Yogosawa, S., Miyauchi, Y., Honda, R., Tanaka, H. & Yasuda, H. Mammalian Numb is a target protein of Mdm2, ubiquitin ligase. Biochem. Biophys. Res. Commun. 302, 869–872 (2003).
Susini, L. et al. Siah-1 binds and regulates the function of Numb. Proc. Natl. Acad. Sci. USA 98, 15067–15072 (2001).
Smith, C.A. et al. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J. 26, 468–480 (2007).
Goldstein, J.L., Basu, S.K. & Brown, M.S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 98, 241–260 (1983).
Brown, A.J., Sun, L., Feramisco, J.D., Brown, M.S. & Goldstein, J.L. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell 10, 237–245 (2002).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Xie, C. et al. Ezetimibe blocks the internalization of NPC1L1 and cholesterol in mouse small intestine. J. Lipid Res. 53, 2092–2101 (2012).
Tang, J.J. et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 13, 44–56 (2011).
Xu, C. et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell 135, 702–713 (2008).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Salisbury, B.G. et al. Hypocholesterolemic activity of a novel inhibitor of cholesterol absorption, SCH 48461. Atherosclerosis 115, 45–63 (1995).
Luo, J. et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2, 1236–1247 (2007).
Acknowledgements
We thank Y.-X. Qu, J. Xu and J. Qin for technical assistance. We thank W. Qi for helpful discussion and revision of the paper. We thank S. Robine (Institut Curie) for the gift of transgenic mice (heterozygous for villin-Cre-ERT2). We thank S. Kathiresan, X. Lin and X.-F. Lu for discussions. This work was supported by grants from the Ministry of Science and Technology of China (2009CB919000, 2011CB910900 and 2012CB524900), National Natural Science Foundation of China (30925012, 31230020, 81260041, 81270155 and 91213306), Shanghai Science and Technology Committee (11JC1414100) and Xinjiang Science and Technology Department (2013911111).
Author information
Authors and Affiliations
Contributions
P.-S.L., C.-Q.X., Y.-T.M., B.-L.L. and B.-L.S. conceived the project. P.-S.L. and B.-L.S. designed the experiments. P.-S.L., Z.-Y.F., Y.-Y.Z. and J.-H.Z. performed the experiments. P.-S.L., C.-Q.X., B.-L.L. and B.-L.S. analyzed the data. P.-S.L. and B.-L.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 2418 kb)
Rights and permissions
About this article
Cite this article
Li, PS., Fu, ZY., Zhang, YY. et al. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat Med 20, 80–86 (2014). https://doi.org/10.1038/nm.3417
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3417
This article is cited by
-
Bile acids-mediated intracellular cholesterol transport promotes intestinal cholesterol absorption and NPC1L1 recycling
Nature Communications (2023)
-
The TICE Pathway: Mechanisms and Potential Clinical Applications
Current Atherosclerosis Reports (2023)
-
Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities
Nature Metabolism (2020)
-
Mechanisms and regulation of cholesterol homeostasis
Nature Reviews Molecular Cell Biology (2020)
-
Regulation of intestinal lipid metabolism: current concepts and relevance to disease
Nature Reviews Gastroenterology & Hepatology (2020)