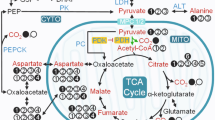
Abstract
Despite the central role of the liver in the regulation of glucose and lipid metabolism, there are currently no methods to directly assess hepatic oxidative metabolism in humans in vivo. By using a new 13C-labeling strategy in combination with 13C magnetic resonance spectroscopy, we show that rates of mitochondrial oxidation and anaplerosis in human liver can be directly determined noninvasively. Using this approach, we found the mean rates of hepatic tricarboxylic acid (TCA) cycle flux (VTCA) and anaplerotic flux (VANA) to be 0.43 ± 0.04 μmol g−1 min−1 and 0.60 ± 0.11 μmol g−1 min−1, respectively, in twelve healthy, lean individuals. We also found the VANA/VTCA ratio to be 1.39 ± 0.22, which is severalfold lower than recently published estimates using an indirect approach. This method will be useful for understanding the pathogenesis of nonalcoholic fatty liver disease and type 2 diabetes, as well as for assessing the effectiveness of new therapies targeting these pathways in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jones, J.G., Solomon, M.A., Cole, S.M., Sherry, A.D. & Malloy, C.R. An integrated 2H and 13C NMR study of gluconeogenesis and TCA cycle flux in humans. Am. J. Physiol. Endocrinol. Metab. 281, E848–E856 (2001).
Jones, J.G., Solomon, M.A., Sherry, A.D., Jeffrey, F.M. & Malloy, C.R. 13C NMR measurements of human gluconeogenic fluxes after ingestion of [U-13C]propionate, phenylacetate, and acetaminophen. Am. J. Physiol. 275, E843–E852 (1998).
Sunny, N.E., Parks, E.J., Browning, J.D. & Burgess, S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 14, 804–810 (2011).
Landau, B.R. et al. 14C-labeled propionate metabolism in vivo and estimates of hepatic gluconeogenesis relative to Krebs cycle flux. Am. J. Physiol. 265, E636–E647 (1993).
Landau, B.R. et al. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J. Clin. Invest. 95, 172–178 (1995).
Shulman, G.I. et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322, 223–228 (1990).
Befroy, D.E. et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56, 1376–1381 (2007).
Lebon, V. et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J. Clin. Invest. 108, 733–737 (2001).
Mason, G.F. et al. Simultaneous determination of the rates of the TCA cycle, glucose utilization, α-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J. Cereb. Blood Flow Metab. 15, 12–25 (1995).
Mason, G.F. et al. Measurement of the tricarboxylic acid cycle rate in human grey and white matter in vivo by 1H-[13C] magnetic resonance spectroscopy at 4.1T. J. Cereb. Blood Flow Metab. 19, 1179–1188 (1999).
Petersen, K.F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
Szendroedi, J. et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 50, 1079–1086 (2009).
Schmid, A.I. et al. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 34, 448–453 (2011).
Befroy, D.E., Rothman, D.L., Petersen, K.F. & Shulman, G.I. 31P-magnetization transfer magnetic resonance spectroscopy measurements of in vivo metabolism. Diabetes 61, 2669–2678 (2012).
Mitchell, C.S. et al. Resistance to thyroid hormone is associated with raised energy expenditure, muscle mitochondrial uncoupling, and hyperphagia. J. Clin. Invest. 120, 1345–1354 (2010).
Boumezbeur, F. et al. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 30, 211–221 (2010).
Lebon, V. et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J. Neurosci. 22, 1523–1531 (2002).
Li, S., Yang, J. & Shen, J. Novel strategy for cerebral 13C MRS using very low RF power for proton decoupling. Magn. Reson. Med. 57, 265–271 (2007).
Li, S. et al. In vivo13C magnetic resonance spectroscopy of human brain on a clinical 3 T scanner using [2-13C]glucose infusion and low-power stochastic decoupling. Magn. Reson. Med. 62, 565–573 (2009).
Sailasuta, N. et al. Clinical NOE 13C MRS for neuropsychiatric disorders of the frontal lobe. J. Magn. Reson. 195, 219–225 (2008).
Befroy, D.E. et al. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc. Natl. Acad. Sci. USA 105, 16701–16706 (2008).
Beylot, M., Soloviev, M.V., David, F., Landau, B.R. & Brunengraber, H. Tracing hepatic gluconeogenesis relative to citric acid cycle activity in vitro and in vivo. Comparisons in the use of [3-13C]lactate, [2-13C]acetate, and α-keto[3-13C]isocaproate. J. Biol. Chem. 270, 1509–1514 (1995).
Puchowicz, M.A. et al. Zonation of acetate labeling across the liver: implications for studies of lipogenesis by MIDA. Am. J. Physiol. 277, E1022–E1027 (1999).
Jin, E.S. et al. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal. Biochem. 327, 149–155 (2004).
Jones, J.G., Carvalho, R.A., Franco, B., Sherry, A.D. & Malloy, C.R. Measurement of hepatic glucose output, Krebs cycle, and gluconeogenic fluxes by NMR analysis of a single plasma glucose sample. Anal. Biochem. 263, 39–45 (1998).
Alves, T.C. et al. Regulation of hepatic fat and glucose oxidation in rats with lipid-induced hepatic insulin resistance. Hepatology 53, 1175–1181 (2011).
Jones, J.G. et al. Measurement of gluconeogenesis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-13C3]propionate. FEBS Lett. 412, 131–137 (1997).
Shen, J., Rycyna, R.E. & Rothman, D.L. Improvements on an in vivo automatic shimming method. Magn. Reson. Med. 38, 834–839 (1997).
Patel, A.B., de Graaf, R.A., Rothman, D.L., Behar, K.L. & Mason, G.F. Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J. Cereb. Blood Flow Metab. 30, 1200–1213 (2010).
Katz, J., Wals, P. & Lee, W.N. Isotopomer studies of gluconeogenesis and the Krebs cycle with 13C-labeled lactate. J. Biol. Chem. 268, 25509–25521 (1993).
Heath, D.F. & Rose, J.G. [14C]bicarbonate fixation into glucose and other metabolites in the liver of the starved rat under halothane anaesthesia. Metabolic channelling of mitochondrial oxaloacetate. Biochem. J. 227, 851–865 (1985).
Petersen, K.F., Blair, J.B. & Shulman, G.I. Triiodothyronine treatment increases substrate cycling between pyruvate carboxylase and malic enzyme in perfused rat liver. Metabolism 44, 1380–1383 (1995).
Fernandez, C.A. & Des Rosiers, C. Modeling of liver citric acid cycle and gluconeogenesis based on 13C mass isotopomer distribution analysis of intermediates. J. Biol. Chem. 270, 10037–10042 (1995).
Acknowledgements
We thank A. Impellizeri, Y. Kosover, I. Smolgovsky, M. Smolgovsky, G. Solomon, C. Parmelee and the staff of the Yale Center for Clinical Investigation Hospital Research Unit for their technical support and the volunteers for their participation in these studies. We also acknowledge the contributions of G. Mason, who assisted in the implementation and interpretation of the metabolic modeling of the rat [1-13C]acetate infusion data. This publication was supported by grants from the US Public Health Service (R24 DK-085638, R01 AG-23686, R01 DK-49230, P30 DK-45735 and UL1 RR-024139), a Distinguished Clinical Investigator Award from the American Diabetes Association (K.F.P.) and an investigator-initiated grant from Pfizer (K.F.P.). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the US National Center for Research Resources or National Institutes of Health.
Author information
Authors and Affiliations
Contributions
D.E.B., R.J.P., J.T., J.B., K.F.P., D.L.R. and G.I.S. designed the experimental protocols. D.E.B., R.J.P., S.D., G.W.C. and K.F.P. performed the studies. D.E.B., N.J., R.J.P., S.D., G.W.C. and D.L.R. analyzed the data. D.E.B., R.J.P., K.F.P., D.L.R. and G.I.S. contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2, Supplementary Figures 1–3 and Supplementary Methods (PDF 4067 kb)
Source data
Rights and permissions
About this article
Cite this article
Befroy, D., Perry, R., Jain, N. et al. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat Med 20, 98–102 (2014). https://doi.org/10.1038/nm.3415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3415
This article is cited by
-
Slow TCA flux and ATP production in primary solid tumours but not metastases
Nature (2023)
-
[13C]bicarbonate labelled from hyperpolarized [1-13C]pyruvate is an in vivo marker of hepatic gluconeogenesis in fasted state
Communications Biology (2022)
-
PHIP hyperpolarized [1-13C]pyruvate and [1-13C]acetate esters via PH-INEPT polarization transfer monitored by 13C NMR and MRI
Scientific Reports (2021)
-
The Role of Mitochondrial Adaptation and Metabolic Flexibility in the Pathophysiology of Obesity and Insulin Resistance: an Updated Overview
Current Obesity Reports (2021)
-
[11C]acetate PET as a tool for diagnosis of liver steatosis
Abdominal Radiology (2018)