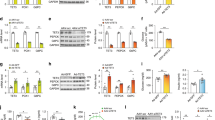
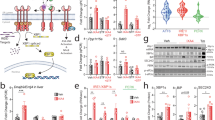
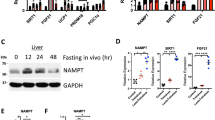
Abstract
Signaling initiated by hypoxia and insulin powerfully alters cellular metabolism. The protein stability of hypoxia-inducible factor-1 alpha (Hif-1α) and Hif-2α is regulated by three prolyl hydroxylase domain–containing protein isoforms (Phd1, Phd2 and Phd3). Insulin receptor substrate-2 (Irs2) is a critical mediator of the anabolic effects of insulin, and its decreased expression contributes to the pathophysiology of insulin resistance and diabetes1. Although Hif regulates many metabolic pathways2, it is unknown whether the Phd proteins regulate glucose and lipid metabolism in the liver. Here, we show that acute deletion of hepatic Phd3, also known as Egln3, improves insulin sensitivity and ameliorates diabetes by specifically stabilizing Hif-2α, which then increases Irs2 transcription and insulin-stimulated Akt activation. Hif-2α and Irs2 are both necessary for the improved insulin sensitivity, as knockdown of either molecule abrogates the beneficial effects of Phd3 knockout on glucose tolerance and insulin-stimulated Akt phosphorylation. Augmenting levels of Hif-2α through various combinations of Phd gene knockouts did not further improve hepatic metabolism and only added toxicity. Thus, isoform-specific inhibition of Phd3 could be exploited to treat type 2 diabetes without the toxicity that could occur with chronic inhibition of multiple Phd isoforms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taniguchi, C.M., Emanuelli, B. & Kahn, C.R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006).
Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 8, 705–713 (2008).
Ivan, M. et al. HIF-α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Min, J.H. et al. Structure of an HIF-1α–pVHL complex: hydroxyproline recognition in signaling. Science 296, 1886–1889 (2002).
Appelhoff, R.J. et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279, 38458–38465 (2004).
Rankin, E.B. et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149, 63–74 (2012).
Taniguchi, C.M., Ueki, K. & Kahn, R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J. Clin. Invest. 115, 718–727 (2005).
Minamishima, Y.A. et al. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol. Cell Biol. 29, 5729–5741 (2009).
Horton, J.D., Goldstein, J.L. & Brown, M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Chen, C., Pore, N., Behrooz, A., Ismail-Beigi, F. & Maity, A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 276, 9519–9525 (2001).
Minamishima, Y.A. et al. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol. Cell Biol. 29, 5729–5741 (2009).
Kim, W.Y. et al. Failure to prolyl hydroxylate hypoxia-inducible factor α phenocopies VHL inactivation in vivo. EMBO J. 25, 4650–4662 (2006).
Puigserver, P. et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423, 550–555 (2003).
Vassen, L., Wegrzyn, W. & Klein-Hitpass, L. Human insulin receptor substrate-2: gene organization and promoter characterization. Diabetes 48, 1877–1880 (1999).
Kim, W.Y. et al. Failure to prolyl hydroxylate hypoxia-inducible factor α phenocopies VHL inactivation in vivo. EMBO J. 25, 4650–4662 (2006).
Nakagawa, Y. et al. TFE3 transcriptionally activates hepatic IRS-2, participates in insulin signaling and ameliorates diabetes. Nat. Med. 12, 107–113 (2006).
Saito, T. et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 16, 678–686 (2010).
Kubota, N et al. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 8, 49–64 (2008).
Canettieri, G. et al. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2, 331–338 (2005).
Takeda, K. et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood 111, 3229–3235 (2008).
Minamishima, Y.A. & Kaelin, W.G. Jr. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science 329, 407 (2010).
Shen, X. et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J. Orthop. Res. 27, 1298–1305 (2009).
Myllyharju, J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann. Med. 40, 402–417 (2008).
Luo, J. et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2, 1236–1247 (2007).
Wei, K. et al. A liver Hif-2α–Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat. Med. 10.1038/nm.3295 (2013).
Rankin, E.B. et al. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease–associated vascular tumors in mice. Mol. Cell Biol. 25, 3163–3172 (2005).
Acknowledgements
We thank K. Takeda and G.-H. Fong (University of Connecticut) for their generous gift of the Phd1fl/fl, Phd2fl/fl and Phd3fl/fl mice. We thank J. Boucher (Joslin Diabetes Center, Boston, MA) for sharing qPCR primer sequences and his critical reading of the manuscript. We thank S. Biddinger (Boston Children's Hospital, Boston, MA) for providing the Fao hepatoma cells. C.M.T. was supported by Radiological Society of North America Research Resident grants 1018 and 1111. E.C.F. and E.L.L. were supported by US National Cancer Institute Training Grant CA121940. C.W. was supported by a training grant from the Canadian Institutes of Health and Research. A.N.D. was supported by a T32 training grant in Comparative Animal Medicine at Stanford University. A.J.K. was supported by grant P20 GM104936 from the US National Institute of General Medical Sciences (NIGMS). Fellowship support was from the NIGMS Stanford Medical Scientist Training Program grant T32 GM007365 (K.W.), Stanford Medical Science Training Program funding (K.W. and L.M.M.), Molecular and Cellular Immunobiology Program training grant 5T32AI07290 (L.M.M.), and US National Institutes of Health (NIH) R01HL074267, R01NS064517 and R01CA158528 (C.J.K.). A.J.G. was supported by NIH grants CA67166 and CA88480 and the Sidney Frank Foundation.
Author information
Authors and Affiliations
Contributions
C.M.T., E.C.F., A.J.K., E.L.L., K.W. and L.M.M. designed and performed experiments and analyzed data. C.W. and A.N.D. generated the knockout animals and contributed to design of all animal experiments. J.Y. and C.J.K. generated and purified the adenoviruses and contributed to experimental design of all adenovirus experiments. C.M.T. and A.J.G. wrote the manuscript and oversaw all aspects of this project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 7831 kb)
Rights and permissions
About this article
Cite this article
Taniguchi, C., Finger, E., Krieg, A. et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat Med 19, 1325–1330 (2013). https://doi.org/10.1038/nm.3294
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3294
This article is cited by
-
PHD1-3 oxygen sensors in vivo—lessons learned from gene deletions
Pflügers Archiv - European Journal of Physiology (2024)
-
Transgenerational exposure to ocean acidification impacts the hepatic transcriptome of European sea bass (Dicentrarchus labrax)
BMC Genomics (2023)
-
Emerging roles of TFE3 in metabolic regulation
Cell Death Discovery (2023)
-
Modelling liver cancer microenvironment using a novel 3D culture system
Scientific Reports (2022)
-
Hypoxia signaling in human health and diseases: implications and prospects for therapeutics
Signal Transduction and Targeted Therapy (2022)