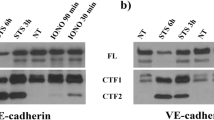
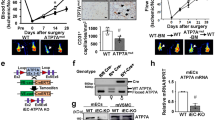
Abstract
Endothelial secretion of von Willebrand factor (VWF) from intracellular organelles known as Weibel-Palade bodies (WPBs) is required for platelet adhesion to the injured vessel wall. Here we demonstrate that WPBs are often found near or within autophagosomes and that endothelial autophagosomes contain abundant VWF protein. Pharmacological inhibitors of autophagy or knockdown of the essential autophagy genes Atg5 or Atg7 inhibits the in vitro secretion of VWF. Furthermore, although mice with endothelial-specific deletion of Atg7 have normal vessel architecture and capillary density, they exhibit impaired epinephrine-stimulated VWF release, reduced levels of high–molecular weight VWF multimers and a corresponding prolongation of bleeding times. Endothelial-specific deletion of Atg5 or pharmacological inhibition of autophagic flux results in a similar in vivo alteration of hemostasis. Thus, autophagy regulates endothelial VWF secretion, and transient pharmacological inhibition of autophagic flux may be a useful strategy to prevent thrombotic events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pfeffer, S.R. Unconventional secretion by autophagosome exocytosis. J. Cell Biol. 188, 451–452 (2010).
Abrahamsen, H. & Stenmark, H. Protein secretion: unconventional exit by exophagy. Curr. Biol. 20, R415–R418 (2010).
Manjithaya, R. & Subramani, S. Autophagy: a broad role in unconventional protein secretion? Trends Cell Biol. 21, 67–73 (2011).
Deretic, V., Jiang, S. & Dupont, N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 22, 397–406 (2012).
Duran, J.M., Anjard, C., Stefan, C., Loomis, W.F. & Malhotra, V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 188, 527–536 (2010).
Manjithaya, R., Anjard, C., Loomis, W.F. & Subramani, S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell Biol. 188, 537–546 (2010).
Dupont, N. et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 30, 4701–4711 (2011).
Narita, M. et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Cadwell, K. et al. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145 (2010).
Ushio, H. et al. Crucial role for autophagy in degranulation of mast cells. J. Allergy Clin. Immunol. 127, 1267–1276.e6 (2011).
Ebato, C. et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 8, 325–332 (2008).
Jung, H.S. et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 8, 318–324 (2008).
Ganesan, A.K. et al. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 4, e1000298 (2008).
DeSelm, C.J. et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell 21, 966–974 (2011).
Mariño, G. et al. Autophagy is essential for mouse sense of balance. J. Clin. Invest. 120, 2331–2344 (2010).
Lowenstein, C.J., Morrell, C.N. & Yamakuchi, M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc. Med. 15, 302–308 (2005).
Valentijn, K.M., Sadler, J.E., Valentijn, J.A., Voorberg, J. & Eikenboom, J. Functional architecture of Weibel-Palade bodies. Blood 117, 5033–5043 (2011).
Denis, C.V., Andre, P., Saffaripour, S. & Wagner, D.D. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc. Natl. Acad. Sci. USA 98, 4072–4077 (2001).
Haberichter, S.L. et al. Re-establishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells. Blood 105, 145–152 (2005).
Wagner, D.D. et al. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell 64, 403–413 (1991).
Voorberg, J. et al. Biogenesis of von Willebrand factor-containing organelles in heterologous transfected CV-1 cells. EMBO J. 12, 749–758 (1993).
Rodeghiero, F., Castaman, G. & Dini, E. Epidemiological investigation of the prevalence of von Willebrand's disease. Blood 69, 454–459 (1987).
Metcalf, D.J., Nightingale, T.D., Zenner, H.L., Lui-Roberts, W.W. & Cutler, D.F. Formation and function of Weibel-Palade bodies. J. Cell Sci. 121, 19–27 (2008).
Wagner, D.D., Mayadas, T., Urban-Pickering, M., Lewis, B.H. & Marder, V.J. Inhibition of disulfide bonding of von Willebrand protein by monensin results in small, functionally defective multimers. J. Cell Biol. 101, 112–120 (1985).
Michaux, G. et al. The physiological function of von Willebrand's factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev. Cell 10, 223–232 (2006).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Komatsu, M. & Ichimura, Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 584, 1374–1378 (2010).
Klionsky, D.J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 (2008).
Jia, W., Pua, H.H., Li, Q.J. & He, Y.W. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J. Immunol. 186, 1564–1574 (2011).
Wagner, D.D. Cell biology of von Willebrand factor. Annu. Rev. Cell Biol. 6, 217–246 (1990).
Wagner, D.D., Mayadas, T. & Marder, V.J. Initial glycosylation and acidic pH in the Golgi apparatus are required for multimerization of von Willebrand factor. J. Cell Biol. 102, 1320–1324 (1986).
Zenner, H.L., Collinson, L.M., Michaux, G. & Cutler, D.F. High-pressure freezing provides insights into Weibel-Palade body biogenesis. J. Cell Sci. 120, 2117–2125 (2007).
Mijaljica, D., Prescott, M. & Devenish, R.J. Endoplasmic reticulum and Golgi complex: Contributions to, and turnover by, autophagy. Traffic 7, 1590–1595 (2006).
Erent, M. et al. Rate, extent and concentration dependence of histamine-evoked Weibel-Palade body exocytosis determined from individual fusion events in human endothelial cells. J. Physiol. (Lond.) 583, 195–212 (2007).
Zhou, Y.F. et al. A pH-regulated dimeric bouquet in the structure of von Willebrand factor. EMBO J. 30, 4098–4111 (2011).
Babich, V. et al. Selective release of molecules from Weibel-Palade bodies during a lingering kiss. Blood 111, 5282–5290 (2008).
Alva, J.A. et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235, 759–767 (2006).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Komatsu, M. et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 (2006).
Kim, J.H., Yu, Y.S., Mun, J.Y. & Kim, K.W. Autophagy-induced regression of hyaloid vessels in early ocular development. Autophagy 6, 922–928 (2010).
Lee, S.J., Kim, H.P., Jin, Y., Choi, A.M. & Ryter, S.W. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy 7, 829–839 (2011).
Kanaji, S., Fahs, S.A., Shi, Q., Haberichter, S.L. & Montgomery, R.R. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J. Thromb. Haemost. 10, 1646–1652 (2012).
Komatsu, M. et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 (2005).
Lee, I.H. et al. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 336, 225–228 (2012).
Wu, J.J. et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 1, 425–437 (2009).
Sirois, I. et al. Caspase activation regulates the extracellular export of autophagic vacuoles. Autophagy 8, 927–937 (2012).
Pallet, N. et al. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 13, 1108–1120 (2013).
Johnson, R. & Charnley, J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin. Orthop. Relat. Res. 174–177 (1979).
Komatsu, M. et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 (2005).
Alva, J.A. et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235, 759–767 (2006).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Tan, W. et al. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 22, 1829–1838 (2008).
Lee, I.H. et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 105, 3374–3379 (2008).
Tokuyasu, K.T. Application of cryoultramicrotomy to immunocytochemistry. J. Microsc. 143, 139–149 (1986).
Zinselmeyer, B.H. et al. Chapter 16. Two-photon microscopy and multidimensional analysis of cell dynamics. Methods Enzymol. 461, 349–378 (2009).
Takaku, T. et al. Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy. Blood 116, e41–e55 (2010).
Pitulescu, M.E., Schmidt, I., Benedito, R. & Adams, R.H. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat. Protoc. 5, 1518–1534 (2010).
Erent, M. et al. Rate, extent and concentration dependence of histamine-evoked Weibel-Palade body exocytosis determined from individual fusion events in human endothelial cells. J. Physiol. (Lond.) 583, 195–212 (2007).
Enayat, M.S. Multimeric analysis of von Willebrand factor. Methods Mol. Med. 31, 187–200 (1999).
Kanaji, S., Fahs, S.A., Shi, Q., Haberichter, S.L. & Montgomery, R.R. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J. Thromb. Haemost. 10, 1646–1652 (2012).
Babich, V. et al. Selective release of molecules from Weibel-Palade bodies during a lingering kiss. Blood 111, 5282–5290 (2008).
Zhao, B.Q. et al. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood 114, 3329–3334 (2009).
Acknowledgements
We are grateful to S. Gutkind (NIH) for help with primary endothelial cell isolation, T. Carter for the gift of the VWF monomeric GFP plasmid (VWF-mGFP), J. Lippincott-Schwartz (NIH) for the LC3-mCherry plasmid and Y. Fitz (NIH) for help with coagulation measurements. We thank B. Zinselmeyer (NIH) for assistance with the spot cluster analysis and C.A. Brantner (NIH) for help performing cryo-immunogold electron microscopy. This work was supported by NIH Intramural Funds. T.T. is a recipient of a Japan Society for the Promotion of Science Research Fellowship in Biomedical and Behavioral Research at the NIH.
Author information
Authors and Affiliations
Contributions
T.T. and K.T. designed, performed and analyzed the experiments and aided in writing the manuscript. I.H.L., J.L., I.I.R., M.M.F. and L.C. contributed to the completion of various experiments. D.M., C.A.C., X.S.W., R.W., P.S.C. and M.P.D. aided in specialized imaging procedures. M.K. provided valuable reagents and advice. T.F. helped conceive the study, supervised the research and contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 1155 kb)
Supplementary Video 1
Three dimensional reconstitution of super resolution images of the interaction of autophagosomes and WPBs. Images of LC3 (green) and VWF (red) are shown with a LC3 coated autophagosome apparently encircling a WPB. (MOV 757 kb)
Supplementary Video 2
Dual-color TIRF imaging of LC3-mCherry and VWF-mGFP following histamine stimulation in HUVEC. (MOV 1182 kb)
Supplementary Video 3
TIRF imaging of LC3-GFP following histamine stimulation in HUVEC. (MOV 4025 kb)
Supplementary Video 4
VWF-GFP analysis following histamine stimulation in control knockdown cells. Arrows indicate WPB fusion events. (MOV 3939 kb)
Supplementary Video 5
VWF-GFP analysis following histamine stimulation in Atg7 knockdown cells. (AVI 154375 kb)
Rights and permissions
About this article
Cite this article
Torisu, T., Torisu, K., Lee, I. et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat Med 19, 1281–1287 (2013). https://doi.org/10.1038/nm.3288
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3288
This article is cited by
-
Angiogenesis-on-a-chip coupled with single-cell RNA sequencing reveals spatially differential activations of autophagy along angiogenic sprouts
Nature Communications (2024)
-
Hallmarks of cardiovascular ageing
Nature Reviews Cardiology (2023)
-
Research Progress on Histone Deacetylases Regulating Programmed Cell Death in Atherosclerosis
Journal of Cardiovascular Translational Research (2023)
-
lncR-GAS5 upregulates the splicing factor SRSF10 to impair endothelial autophagy, leading to atherogenesis
Frontiers of Medicine (2023)
-
Autophagy protein 5 controls flow-dependent endothelial functions
Cellular and Molecular Life Sciences (2023)