Abstract
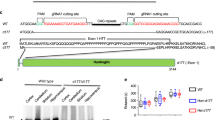
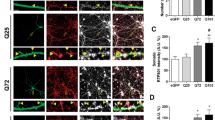
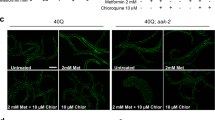
Huntington's disease is caused by an expanded polyglutamine repeat in the huntingtin protein (HTT), but the pathophysiological sequence of events that trigger synaptic failure and neuronal loss are not fully understood. Alterations in N-methyl-D-aspartate (NMDA)-type glutamate receptors (NMDARs) have been implicated. Yet, it remains unclear how the HTT mutation affects NMDAR function, and direct evidence for a causative role is missing. Here we show that mutant HTT redirects an intracellular store of juvenile NMDARs containing GluN3A subunits to the surface of striatal neurons by sequestering and disrupting the subcellular localization of the endocytic adaptor PACSIN1, which is specific for GluN3A. Overexpressing GluN3A in wild-type mouse striatum mimicked the synapse loss observed in Huntington's disease mouse models, whereas genetic deletion of GluN3A prevented synapse degeneration, ameliorated motor and cognitive decline and reduced striatal atrophy and neuronal loss in the YAC128 Huntington's disease mouse model. Furthermore, GluN3A deletion corrected the abnormally enhanced NMDAR currents, which have been linked to cell death in Huntington's disease and other neurodegenerative conditions. Our findings reveal an early pathogenic role of GluN3A dysregulation in Huntington's disease and suggest that therapies targeting GluN3A or pathogenic HTT-PACSIN1 interactions might prevent or delay disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
DiFiglia, M. et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 14, 1075–1081 (1995).
Schaffar, G. et al. Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol. Cell 15, 95–105 (2004).
Kaltenbach, L.S. et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 3, e82 (2007).
Goehler, H. et al. A protein interaction network links GIT1, an enhancer of huntingtin aggregation, to Huntington's disease. Mol. Cell 15, 853–865 (2004).
Qin, Z.H. et al. Huntingtin bodies sequester vesicle-associated proteins by a polyproline-dependent interaction. J. Neurosci. 24, 269–281 (2004).
Li, J.Y., Plomann, M. & Brundin, P. Huntington's disease: a synaptopathy? Trends Mol. Med. 9, 414–420 (2003).
Pérez-Otaño, I. et al. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat. Neurosci. 9, 611–621 (2006).
Levine, M.S. et al. Enhanced sensitivity to N-methyl-d-aspartate receptor activation in transgenic and knock-in mouse models of Huntington's disease. J. Neurosci. Res. 58, 515–532 (1999).
Zeron, M.M. et al. Increased sensitivity to N-methyl-d-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33, 849–860 (2002).
Cepeda, C. et al. NMDA receptor function in mouse models of Huntington disease. J. Neurosci. Res. 66, 525–539 (2001).
Laforet, G.A. et al. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington's disease. J. Neurosci. 21, 9112–9123 (2001).
Milnerwood, A.J. et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice. Neuron 65, 178–190 (2010).
Beal, M.F. et al. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 321, 168–171 (1986).
Das, S. et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393, 377–381 (1998).
Roberts, A.C. et al. Downregulation of NR3A-containing NMDARs is required for synapse maturation and memory consolidation. Neuron 63, 342–356 (2009).
Modregger, J., DiProspero, N.A., Charles, V., Tagle, D.A. & Plomann, M. PACSIN 1 interacts with huntingtin and is absent from synaptic varicosities in presymptomatic Huntington's disease brains. Hum. Mol. Genet. 11, 2547–2558 (2002).
Lim, J. et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature 452, 713–718 (2008).
Slow, E.J. et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum. Mol. Genet. 12, 1555–1567 (2003).
Arrasate, M., Mitra, S., Schweitzer, E.S., Segal, M.R. & Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810 (2004).
Li, Q. et al. A syntaxin 1, Gα(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J. Neurosci. 24, 4070–4081 (2004).
Perez-Otaño, I. et al. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J. Neurosci. 21, 1228–1237 (2001).
Li, X. et al. Mutant huntingtin impairs vesicle formation from recycling endosomes by interfering with Rab11 activity. Mol. Cell Biol. 29, 6106–6116 (2009).
Park, M., Penick, E.C., Edwards, J.G., Kauer, J.A. & Ehlers, M.D. Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972–1975 (2004).
Wong, H.K. et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J. Comp. Neurol. 450, 303–317 (2002).
Henson, M.A., Roberts, A.C., Perez-Otaño, I. & Philpot, B.D. Influence of the NR3A subunit on NMDA receptor functions. Prog. Neurobiol. 91, 23–37 (2010).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996).
Qualmann, B., Roos, J., DiGregorio, P.J. & Kelly, R.B. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol. Biol. Cell 10, 501–513 (1999).
Canals, J.M. et al. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J. Neurosci. 24, 7727–7739 (2004).
Mazarakis, N.K. et al. Deficits in experience-dependent cortical plasticity and sensory-discrimination learning in presymptomatic Huntington's disease mice. J. Neurosci. 25, 3059–3066 (2005).
Van Raamsdonk, J.M. et al. Phenotypic abnormalities in the YAC128 mouse model of Huntington disease are penetrant on multiple genetic backgrounds and modulated by strain. Neurobiol. Dis. 26, 189–200 (2007).
Graham, R.K. et al. Levels of mutant huntingtin influence the phenotypic severity of Huntington disease in YAC128 mouse models. Neurobiol. Dis. 21, 444–455 (2006).
Wheeler, V.C. et al. Early phenotypes that presage late-onset neurodegenerative disease allow testing of modifiers in Hdh CAG knock-in mice. Hum. Mol. Genet. 11, 633–640 (2002).
Martínez-Turrillas, R. et al. The NMDA receptor subunit GluN3A protects against 3-nitroproprionic–induced striatal lesions via inhibition of calpain activation. Neurobiol. Dis. 48, 290–298 (2012).
Graveland, G.A., Williams, R.S. & DiFiglia, M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington's disease. Science 227, 770–773 (1985).
Cummings, D.M., Cepeda, C. & Levine, M.S. Alterations in striatal synaptic transmission are consistent across genetic mouse models of Huntington's disease. ASN Neuro 2, e00036 (2010).
Van Raamsdonk, J.M. et al. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J. Neurosci. 25, 4169–4180 (2005).
Bibb, J.A. et al. Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proc. Natl. Acad. Sci. USA 97, 6809–6814 (2000).
Vonsattel, J.P. Huntington disease models and human neuropathology: similarities and differences. Acta Neuropathol. 115, 55–69 (2008).
Rudnicki, D.D., Pletnikova, O., Vonsattel, J.P., Ross, C.A. & Margolis, R.L. A comparison of huntington disease and huntington disease–like 2 neuropathology. J. Neuropathol. Exp. Neurol. 67, 366–374 (2008).
Reinhart, P.H. et al. Identification of anti-inflammatory targets for Huntington's disease using a brain slice-based screening assay. Neurobiol. Dis. 43, 248–256 (2011).
Slow, E.J. et al. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc. Natl. Acad. Sci. USA 102, 11402–11407 (2005).
Okamoto, S. et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med. 15, 1407–1413 (2009).
Bodner, R.A. et al. Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases. Proc. Natl. Acad. Sci. USA 103, 4246–4251 (2006).
Li, L., Murphy, T.H., Hayden, M.R. & Raymond, L.A. Enhanced striatal NR2B-containing N-methyl-d-aspartate receptor–mediated synaptic currents in a mouse model of Huntington disease. J. Neurophysiol. 92, 2738–2746 (2004).
Murphy, K.P. et al. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation. J. Neurosci. 20, 5115–5123 (2000).
Usdin, M.T., Shelbourne, P.F., Myers, R.M. & Madison, D.V. Impaired synaptic plasticity in mice carrying the Huntington's disease mutation. Hum. Mol. Genet. 8, 839–846 (1999).
Lynch, G. et al. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease. J. Neurosci. 27, 4424–4434 (2007).
Starling, A.J. et al. Alterations in N-methyl-D-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington's disease. J. Neurosci. Res. 82, 377–386 (2005).
Hardingham, G.E. & Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696 (2010).
Cepeda, C., Wu, N., Andre, V.M., Cummings, D.M. & Levine, M.S. The corticostriatal pathway in Huntington's disease. Prog. Neurobiol. 81, 253–271 (2007).
Nakanishi, N. et al. Neuroprotection by the NR3A subunit of the NMDA receptor. J. Neurosci. 29, 5260–5265 (2009).
Hansson, O. et al. Transgenic mice expressing a Huntington's disease mutation are resistant to quinolinic acid–induced striatal excitotoxicity. Proc. Natl. Acad. Sci. USA 96, 8727–8732 (1999).
Graham, R.K. et al. Differential susceptibility to excitotoxic stress in YAC128 mouse models of Huntington disease between initiation and progression of disease. J. Neurosci. 29, 2193–2204 (2009).
Irwin, S. et al. RNA association and nucleocytoplasmic shuttling by ataxin-1. J. Cell Sci. 118, 233–242 (2005).
Lloret, A. et al. Genetic background modifies nuclear mutant huntingtin accumulation and HD CAG repeat instability in Huntington's disease knock-in mice. Hum. Mol. Genet. 15, 2015–2024 (2006).
Grosshans, D.R., Clayton, D.A., Coultrap, S.J. & Browning, M.D. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci. STKE 2002, Pl8 (2002).
Giralt, A., Carreton, O., Lao-Peregrin, C., Martin, E.D. & Alberch, J. Conditional BDNF release under pathological conditions improves Huntington's disease pathology by delaying neuronal dysfunction. Mol. Neurodegener. 6, 71 (2011).
Ricobaraza, A., Cuadrado-Tejedor, M., Marco, S., Perez-Otano, I. & Garcia-Osta, A. Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus 22, 1040–1050 (2012).
Acknowledgements
We thank S. Finkbeiner (Gladstone Institute, University of California San Francisco), H. Zoghbi (Baylor College of Medicine), T. Yamamoto (University of Tokyo) and M. Ehlers (Pfizer Neuroscience) for providing reagents, A. Zandueta, C. Rodríguez-Viña, F. Ballesteros, X. Remírez and M. Montañana for excellent technical help, M. Galarraga for advice with image analysis, and M. Ehlers, M. Arrasate, T. Aragón, J.A. Esteban and B.D. Philpot for critical readings of the manuscript. This work was funded by the Unión Temporal de Empresas (UTE) project at the Centro de Investigación Médica Aplicada, Gobierno de Navarra, and Spanish Ministry of Science grants (SAF2010-20636 and CSD2008-00005 to I.P.-O., BFU2009-12160 to J.F.W. and SAF2011-29507 to J.A.), grants from the Hereditary Disease Foundation (to I.P.-O. and D.C.L.), US National Institutes of Health grants P01 HD29587, P01 ES016738 and R01 EY05477 (to S.A.L.) and grants from the Cure Huntington's Disease Initiative and the Canadian Institutes of Health Research (to M.R.H.). M.R.H., a Killam University Professor, holds a Canada Research Chair in Human Genetics.
Author information
Authors and Affiliations
Contributions
S.M. performed and analyzed the biochemical experiments in cultured neurons, recombinant cells and YAC128 mice, the imaging experiments in cultured neurons and the spine morphology measurements and collaborated with A.G. on neuropathological studies. A.G. performed and analyzed biochemical experiments in humans and R6/1 and knock-in mice and the behavioral experiments. M.M.P. performed electrophysiological recordings. M.A.P. performed and analyzed behavioral experiments. R.M.-T. contributed to mouse genotyping and conducted biochemical fractionation and real-time PCR assays. J.M.-H. and R.L. performed and analyzed electron microscopy studies. L.S.K. and D.C.L. performed and analyzed slice culture experiments. J.T.-P. performed the initial biochemical experiments in R6/1 mice. M.R.H. and R.K.G. provided the YAC128 mice. N.N. and S.A.L. provided the Grin3a−/− mice. M.W. made the GluN3A-specific antibody used in biochemical and immunocytochemical analyses. J.A. designed and supervised experiments. J.F.W. designed and analyzed electrophysiological and fluorescence colocalization experiments and contributed to the manuscript writing. I.P.-O. conceived the study, designed experiments, analyzed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 3101 kb)
Rights and permissions
About this article
Cite this article
Marco, S., Giralt, A., Petrovic, M. et al. Suppressing aberrant GluN3A expression rescues synaptic and behavioral impairments in Huntington's disease models. Nat Med 19, 1030–1038 (2013). https://doi.org/10.1038/nm.3246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3246
This article is cited by
-
Mitochondrial Abnormalities and Synaptic Damage in Huntington’s Disease: a Focus on Defective Mitophagy and Mitochondria-Targeted Therapeutics
Molecular Neurobiology (2021)
-
Synaptic RTP801 contributes to motor-learning dysfunction in Huntington’s disease
Cell Death & Disease (2020)
-
Structural features in the glycine-binding sites of the GluN1 and GluN3A subunits regulate the surface delivery of NMDA receptors
Scientific Reports (2019)
-
Cell-Autonomous and Non-cell-Autonomous Pathogenic Mechanisms in Huntington's Disease: Insights from In Vitro and In Vivo Models
Neurotherapeutics (2019)
-
Altering cortical input unmasks synaptic phenotypes in the YAC128 cortico-striatal co-culture model of Huntington disease
BMC Biology (2018)