Abstract
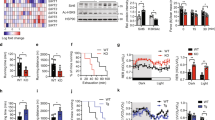
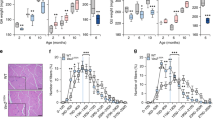
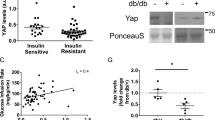
A shift from oxidative to glycolytic metabolism has been associated with skeletal muscle insulin resistance in type 2 diabetes1,2,3,4,5. However, whether this metabolic switch is deleterious or adaptive remains under debate6,7,8, in part because of a limited understanding of the regulatory network that directs the metabolic and contractile specification of fast-twitch glycolytic muscle. Here we show that Baf60c (also called Smarcd3), a transcriptional cofactor enriched in fast-twitch muscle, promotes a switch from oxidative to glycolytic myofiber type through DEP domain–containing mTOR-interacting protein (Deptor)-mediated Akt activation. Muscle-specific transgenic expression of Baf60c activates a program of molecular, metabolic and contractile changes characteristic of glycolytic muscle. In addition, Baf60c is required for maintaining glycolytic capacity in adult skeletal muscle in vivo. Baf60c expression is significantly lower in skeletal muscle from obese mice compared to that from lean mice. Activation of the glycolytic muscle program by transgenic expression of Baf60c protects mice from diet-induced insulin resistance and glucose intolerance. Further mechanistic studies revealed that Deptor is induced by the Baf60c-Six4 transcriptional complex and mediates activation of Akt and glycolytic metabolism by Baf60c in a cell-autonomous manner. This work defines a fundamental mechanism underlying the specification of fast-twitch glycolytic muscle and illustrates that the oxidative-to-glycolytic metabolic shift in skeletal muscle is potentially adaptive and beneficial in the diabetic state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Kelley, D.E., He, J., Menshikova, E.V. & Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950 (2002).
Lillioja, S. et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J. Clin. Invest. 80, 415–424 (1987).
Mootha, V.K. et al. PGC-1α–responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Patti, M.E. et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 100, 8466–8471 (2003).
Petersen, K.F., Dufour, S., Befroy, D., Garcia, R. & Shulman, G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 350, 664–671 (2004).
Muoio, D.M. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim. Biophys. Acta 1801, 281–288 (2010).
Turner, N. & Heilbronn, L.K. Is mitochondrial dysfunction acause of insulin resistance? Trends Endocrinol. Metab. 19, 324–330 (2008).
Muoio, D.M. & Neufer, P.D. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 15, 595–605 (2012).
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 (2011).
Bassel-Duby, R. & Olson, E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 75, 19–37 (2006).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Lin, J. et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002).
Lavery, G.G. et al. Deletion of hexose-6-phosphate dehydrogenase activates the unfolded protein response pathway and induces skeletal myopathy. J. Biol. Chem. 283, 8453–8461 (2008).
Wu, J.I., Lessard, J. & Crabtree, G.R. Understanding the words of chromatin regulation. Cell 136, 200–206 (2009).
Choi, C.S. et al. Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl. Acad. Sci. USA 105, 19926–19931 (2008).
Miura, S., Kai, Y., Ono, M. & Ezaki, O. Overexpression of peroxisome proliferator-activated receptor gamma coactivator-1α down-regulates GLUT4 mRNA in skeletal muscles. J. Biol. Chem. 278, 31385–31390 (2003).
Gordon, B.A., Benson, A.C., Bird, S.R. & Fraser, S.F. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res. Clin. Pract. 83, 157–175 (2009).
LeBrasseur, N.K., Walsh, K. & Arany, Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 300, E3–E10 (2011).
Izumiya, Y. et al. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 7, 159–172 (2008).
Peterson, T.R. et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137, 873–886 (2009).
Niro, C. et al. Six1 and Six4 gene expression is necessary to activate the fast-type muscle gene program in the mouse primary myotome. Dev. Biol. 338, 168–182 (2010).
Civitarese, A.E. et al. Regulation of skeletal muscle oxidative capacity and insulin signaling by the mitochondrial rhomboid protease PARL. Cell Metab. 11, 412–426 (2010).
Nalbantoglu, J. et al. Muscle-specific overexpression of the adenovirus primary receptor CAR overcomes low efficiency of gene transfer to mature skeletal muscle. J. Virol. 75, 4276–4282 (2001).
Forcales, S.V. et al. Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J. 31, 301–316 (2012).
Lickert, H. et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432, 107–112 (2004).
Li, S. et al. Genome-wide coactivation analysis of PGC-1α identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab. 8, 105–117 (2008).
Banks, A.S. et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 8, 333–341 (2008).
Narkar, V.A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Timmers, S. et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 (2011).
Akpan, I. et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int. J. Obes. (Lond.) 33, 1265–1273 (2009).
Sternberg, E.A. et al. Identification of upstream and intragenic regulatory elements that confer cell-type–restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol. Cell Biol. 8, 2896–2909 (1988).
Waters, R.E., Rotevatn, S., Li, P., Annex, B.H. & Yan, Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 287, C1342–C1348 (2004).
Dunn, S.E. & Michel, R.N. Coordinated expression of myosin heavy chain isoforms and metabolic enzymes within overloaded rat muscle fibers. Am. J. Physiol. 273, C371–C383 (1997).
Chen, H. et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141, 280–289 (2010).
Kim, J.K. et al. PKC-θ knockout mice are protected from fat-induced insulin resistance. J. Clin. Invest. 114, 823–827 (2004).
Gan, Z. et al. The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 25, 2619–2630 (2011).
Aragonés, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 40, 170–180 (2008).
Hughes, S.D., Quaade, C., Johnson, J.H., Ferber, S. & Newgard, C.B. Transfection of AtT-20ins cells with GLUT-2 but not GLUT-1 confers glucose-stimulated insulin secretion. Relationship to glucose metabolism. J. Biol. Chem. 268, 15205–15212 (1993).
Acknowledgements
We thank S. Gu and C. Rui for assistance in experiments and lab members for discussion. We thank J. Nalbantoglu and P.C. Holland (McGill University) for the gift of the MCK-CAR transgenic mouse strain. We thank the staff at the University of Michigan Transgenic Animal Core for the generation of MCK-Baf60c transgenic mice and D. Sorenson for help with electron microscopy study and acknowledge support from the Michigan Diabetes Research and Training Center (DK020572) and the Nutrition Obesity Research Center (DK089503). This work was supported by the US National Institutes of Health (NIH) (DK095151 and DK077086 to J.D.L.). Z.-X.M. and S.L. are supported by a Postdoctoral Fellowship and a Scientist Development Grant from the American Heart Association, respectively. Clamp studies were performed at the University of Massachusetts Mouse Metabolic Phenotyping Center and were supported by the NIH (U24-DK093000 and R01-DK080756 to J.K.K.). M.O. and Z.Y. are supported by the NIH (AR050429).
Author information
Authors and Affiliations
Contributions
J.D.L. and Z.-X.M. conceived the project and designed research. Z.-X.M., S.L. and L.W. performed the studies. H.J.K., Y.L., D.Y.J. and J.K.K. performed hyperinsulinemic-euglycemic clamp studies. M.O. and Z.Y. performed muscle fiber typing. Z.-X.M. and J.D.L. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 844 kb)
Rights and permissions
About this article
Cite this article
Meng, ZX., Li, S., Wang, L. et al. Baf60c drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Nat Med 19, 640–645 (2013). https://doi.org/10.1038/nm.3144
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3144
This article is cited by
-
Daytime-restricted feeding enhances running endurance without prior exercise in mice
Nature Metabolism (2023)
-
Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study
Signal Transduction and Targeted Therapy (2023)
-
Lunar gravity prevents skeletal muscle atrophy but not myofiber type shift in mice
Communications Biology (2023)
-
FHL3 promotes the formation of fast glycolytic muscle fibers by interacting with YY1 and muscle glycolytic metabolism
Cellular and Molecular Life Sciences (2023)
-
The muscle-enriched myokine Musclin impairs beige fat thermogenesis and systemic energy homeostasis via Tfr1/PKA signaling in male mice
Nature Communications (2023)