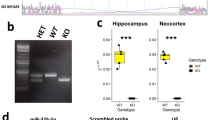
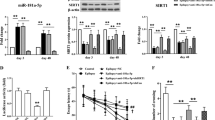
Abstract
Temporal lobe epilepsy is a common, chronic neurological disorder characterized by recurrent spontaneous seizures. MicroRNAs (miRNAs) are small, noncoding RNAs that regulate post-transcriptional expression of protein-coding mRNAs, which may have key roles in the pathogenesis of neurological disorders. In experimental models of prolonged, injurious seizures (status epilepticus) and in human epilepsy, we found upregulation of miR-134, a brain-specific, activity-regulated miRNA that has been implicated in the control of dendritic spine morphology. Silencing of miR-134 expression in vivo using antagomirs reduced hippocampal CA3 pyramidal neuron dendrite spine density by 21% and rendered mice refractory to seizures and hippocampal injury caused by status epilepticus. Depletion of miR-134 after status epilepticus in mice reduced the later occurrence of spontaneous seizures by over 90% and mitigated the attendant pathological features of temporal lobe epilepsy. Thus, silencing miR-134 exerts prolonged seizure-suppressant and neuroprotective actions; determining whether these are anticonvulsant effects or are truly antiepileptogenic effects requires additional experimentation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pitkänen, A. & Lukasiuk, K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 10, 173–186 (2011).
DeFelipe, J. Chandelier cells and epilepsy. Brain 122, 1807–1822 (1999).
McNamara, J.O., Huang, Y.Z. & Leonard, A.S. Molecular signaling mechanisms underlying epileptogenesis. Sci. STKE 2006, re12 (2006).
Wetherington, J., Serrano, G. & Dingledine, R. Astrocytes in the epileptic brain. Neuron 58, 168–178 (2008).
Vezzani, A., French, J., Bartfai, T. & Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 7, 31–40 (2011).
Eacker, S.M., Dawson, T.M. & Dawson, V.L. Understanding microRNAs in neurodegeneration. Nat. Rev. Neurosci. 10, 837–841 (2009).
Saugstad, J.A. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J. Cereb. Blood Flow Metab. 30, 1564–1576 (2010).
Aronica, E. et al. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur. J. Neurosci. 31, 1100–1107 (2010).
Song, Y.J. et al. Temporal lobe epilepsy induces differential expression of hippocampal miRNAs including let-7e and miR-23a/b. Brain Res. 1387, 134–140 (2011).
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 (2002).
Schratt, G.M. et al. A brain-specific microRNA regulates dendritic spine development. Nature 439, 283–289 (2006).
Matsuzaki, M., Honkura, N., Ellis-Davies, G.C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004).
Zhou, Q., Homma, K.J. & Poo, M.M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44, 749–757 (2004).
Noguchi, J., Matsuzaki, M., Ellis-Davies, G.C. & Kasai, H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 46, 609–622 (2005).
Müller, M., Gahwiler, B.H., Rietschin, L. & Thompson, S.M. Reversible loss of dendritic spines and altered excitability after chronic epilepsy in hippocampal slice cultures. Proc. Natl. Acad. Sci. USA 90, 257–261 (1993).
Rensing, N. et al. In vivo imaging of dendritic spines during electrographic seizures. Ann. Neurol. 58, 888–898 (2005).
Zeng, L.H. et al. Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J. Neurosci. 27, 11604–11613 (2007).
Penzes, P., Cahill, M.E., Jones, K.A., VanLeeuwen, J.E. & Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 14, 285–293 (2011).
Bhatt, D.H., Zhang, S. & Gan, W.B. Dendritic spine dynamics. Annu. Rev. Physiol. 71, 261–282 (2009).
Halpain, S., Hipolito, A. & Saffer, L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18, 9835–9844 (1998).
Saneyoshi, T., Fortin, D.A. & Soderling, T.R. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr. Opin. Neurobiol. 20, 108–115 (2010).
Meng, Y. et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron 35, 121–133 (2002).
Christensen, M., Larsen, L.A., Kauppinen, S. & Schratt, G. Recombinant adeno-associated virus-mediated microRNA delivery into the postnatal mouse brain reveals a role for miR-134 in dendritogenesis in vivo. Front Neural Circuits 3, 16 (2010).
Gao, J. et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466, 1105–1109 (2010).
Stark, K.L. et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 40, 751–760 (2008).
Segal, M. Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur. J. Neurosci. 31, 2178–2184 (2010).
Sierra-Paredes, G., Oreiro-Garcia, T., Nunez-Rodriguez, A., Vazquez-Lopez, A. & Sierra-Marcuno, G. Seizures induced by in vivo latrunculin a and jasplakinolide microperfusion in the rat hippocampus. J. Mol. Neurosci. 28, 151–160 (2006).
Meller, R. et al. Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J. Neurosci. 28, 50–59 (2008).
Mouri, G. et al. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 1213, 140–151 (2008).
Murphy, B.M. et al. Contrasting patterns of Bim induction and neuroprotection in Bim-deficient mice between hippocampus and neocortex after status epilepticus. Cell Death Differ. 17, 459–468 (2010).
Jimenez-Mateos, E.M., Mouri, G., Conroy, R.M. & Henshall, D.C. Epileptic tolerance is associated with enduring neuroprotection and uncoupling of the relationship between CA3 damage, neuropeptide Y rearrangement and spontaneous seizures following intra-amygdala kainic acid-induced status epilepticus in mice. Neuroscience 171, 556–565 (2010).
Peters, L. & Meister, G. Argonaute proteins: mediators of RNA silencing. Mol. Cell 26, 611–623 (2007).
Karginov, F.V. et al. A biochemical approach to identifying microRNA targets. Proc. Natl. Acad. Sci. USA 104, 19291–19296 (2007).
Jimenez-Mateos, E.M. et al. MicroRNA expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am. J. Pathol. 179, 2519–2532 (2011).
Krützfeldt, J. et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature 438, 685–689 (2005).
Krützfeldt, J. et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 35, 2885–2892 (2007).
Elmén, J. et al. LNA-mediated microRNA silencing in non-human primates. Nature 452, 896–899 (2008).
Engel, T. et al. Reduced hippocampal damage and epileptic seizures after status epilepticus in mice lacking proapoptotic Puma. FASEB J. 24, 853–861 (2010).
O'Tuathaigh, C.M. et al. Phenotypic characterization of spatial cognition and social behavior in mice with 'knockout' of the schizophrenia risk gene neuregulin 1. Neuroscience 147, 18–27 (2007).
Merino-Serrais, P., Knafo, S., Alonso-Nanclares, L., Fernaud-Espinosa, I. & Defelipe, J. Layer-specific alterations to CA1 dendritic spines in a mouse model of Alzheimer's disease. Hippocampus 21, 1037–1044 (2011).
Ballesteros-Yáñez, I., Benavides-Piccione, R., Bourgeois, J.P., Changeux, J.P. & DeFelipe, J. Alterations of cortical pyramidal neurons in mice lacking high-affinity nicotinic receptors. Proc. Natl. Acad. Sci. USA 107, 11567–11572 (2010).
Engel, T. et al. Loss of p53 results in protracted electrographic seizures and development of an aggravated epileptic phenotype following status epilepticus. Cell Death Dis. 1, e79 (2010).
Shinoda, S. et al. Development of a model of seizure-induced hippocampal injury with features of programmed cell death in the BALB/c mouse. J. Neurosci. Res. 76, 121–128 (2004).
Pitkänen, A., Kharatishvili, I., Narkilahti, S., Lukasiuk, K. & Nissinen, J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 63, 27–42 (2005).
Nairismägi, J. et al. Progression of brain damage after status epilepticus and its association with epileptogenesis: a quantitative MRI study in a rat model of temporal lobe epilepsy. Epilepsia 45, 1024–1034 (2004).
Mathern, G.W., Babb, T.L. & Armstrong, D.L. Hippocampal sclerosis. in Epilepsy: A Comprehensive Textbook Vol. 13 (eds. Engel, J.J. & Pedley, T.A.) 133–155 (Lippincott-Raven Publishers, Philadelphia, 1997).
Cavazos, J.E., Golarai, G. & Sutula, T.P. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J. Neurosci. 11, 2795–2803 (1991).
Hu, K. et al. Expression profile of microRNAs in rat hippocampus following lithium-pilocarpine-induced status epilepticus. Neurosci. Lett. 488, 252–257 (2011).
Tao, J. et al. Deletion of astroglial dicer causes non–cell-autonomous neuronal dysfunction and degeneration. J. Neurosci. 31, 8306–8319 (2011).
Araki, T., Simon, R.P., Taki, W., Lan, J.Q. & Henshall, D.C. Characterization of neuronal death induced by focally evoked limbic seizures in the C57BL/6 mouse. J. Neurosci. Res. 69, 614–621 (2002).
Lee, B. et al. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J. Neurochem. 108, 1251–1265 (2009).
Brandt, C., Potschka, H., Loscher, W. & Ebert, U. N-methyl-D-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in kainate model of temporal lobe epilepsy. Neuroscience 118, 727–740 (2003).
Jimenez-Mateos, E.M. et al. Hippocampal transcriptome after status epilepticus in mice rendered seizure damage-tolerant by epileptic preconditioning features suppressed calcium and neuronal excitability pathways. Neurobiol. Dis. 32, 442–453 (2008).
Nägerl, U.V., Eberhorn, N., Cambridge, S.B. & Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44, 759–767 (2004).
Kim, C.H. & Lisman, J.E. A role of actin filament in synaptic transmission and long-term potentiation. J. Neurosci. 19, 4314–4324 (1999).
Pavlowsky, A. et al. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr. Biol. 20, 103–115 (2010).
Wermeling, D.P. Intranasal delivery of antiepileptic medications for treatment of seizures. Neurotherapeutics 6, 352–358 (2009).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates 2nd edn. (Elsevier, San Diego, California, 2001).
Acknowledgements
We would like to thank J. Varley, J. Phillips and members of the Epilepsy Programme, Beaumont Hospital. We thank S. Miller-Delaney for assistance and N. Plesnila, J. Prehn and R. Simon for helpful suggestions on the manuscript. We thank the Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, Maryland. This work was supported by funding from Science Foundation Ireland awards 08/IN1/B1875 (D.C.H., E.M.J.-M., K.T., G.M. and T.S.), 11/TIDA/B1988 (D.C.H.) and 07/IN.1/B960 (J.W. and C.O.), US National Institute of Neurological Disorders and Stroke award R56 073714 (D.C.H.), an Irish Research Council for Science, Engineering and Technology postdoctoral fellowship (E.M.J.-M.), Irish Health Research Board grant PHD/2007/11 (R.C.M.) and the Spanish Ministry of Education, Science and Innovation grant SAF2009-09394 (J.D.).
Author information
Authors and Affiliations
Contributions
E.M.J.-M. performed expression analyses, tissue culture, spine imaging, histology and epilepsy monitoring. T.E., K.T., G.M. and T.S. performed mouse modeling and telemetry. P.M.-S. and J.D. performed spine injections and data analysis. R.C.M. and S.P. performed expression studies. C.O. and J.L.W. conducted and analyzed the behavioral studies. N.D., D.F.O. and M.A.F. organized the human studies. R.M.C. performed statistical analyses. R.L.S. contributed to study design and analysis. D.C.H. and E.M.J.-M. conceived of the study, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 852 kb)
Rights and permissions
About this article
Cite this article
Jimenez-Mateos, E., Engel, T., Merino-Serrais, P. et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 18, 1087–1094 (2012). https://doi.org/10.1038/nm.2834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2834
This article is cited by
-
Circulating microRNAs from plasma as preclinical biomarkers of epileptogenesis and epilepsy
Scientific Reports (2024)
-
Evaluation of P-glycoprotein-targeting circulating microRNAs as peripheral biomarkers for medically intractable epilepsy
Acta Epileptologica (2023)
-
microRNA profilings identify plasma biomarkers and targets associated with pediatric epilepsy patients
Pediatric Research (2023)
-
The 100 most-cited manuscripts in epilepsy epigenetics: a bibliometric analysis
Child's Nervous System (2023)
-
miR-26b Targets CEP135 Gene to Regulate Nasopharyngeal Carcinoma Proliferation and Migration by NF-κB Pathway
Molecular Biotechnology (2023)