Abstract
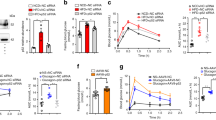
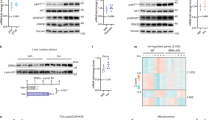
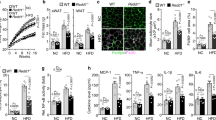
The canonical inhibitor of nuclear factor κB kinase subunit β (IKK-β)–nuclear factor of κ light polypeptide gene enhancer in B cells 1 (NF-κB1) pathway has been well documented to promote insulin resistance; however, the noncanonical NF-κB–inducing kinase (NIK)–NF-κB2 pathway is not well understood in obesity. Additionally, the contribution of counter-regulatory hormones, particularly glucagon, to hyperglycemia in obesity is unclear. Here we show that NIK promotes glucagon responses in obesity. Hepatic NIK was abnormally activated in mice with dietary or genetic obesity. Systemic deletion of Map3k14, encoding NIK, resulted in reduced glucagon responses and hepatic glucose production (HGP). Obesity is associated with high glucagon responses, and liver-specific inhibition of NIK led to lower glucagon responses and HGP and protected against hyperglycemia and glucose intolerance in obese mice. Conversely, hepatocyte-specific overexpression of NIK resulted in higher glucagon responses and HGP. In isolated mouse livers and primary hepatocytes, NIK also promoted glucagon action and glucose production, at least in part by increasing cAMP response element-binding (CREB) stability. Therefore, overactivation of liver NIK in obesity promotes hyperglycemia and glucose intolerance by increasing the hyperglycemic response to glucagon and other factors that activate CREB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Shoelson, S.E. & Goldfine, A.B. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat. Med. 15, 373–374 (2009).
Arkan, M.C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Yuan, M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293, 1673–1677 (2001).
Unger, R.H. & Cherrington, A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 122, 4–12 (2012).
Jiang, G. & Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 284, E671–E678 (2003).
Denroche, H.C. et al. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes 60, 1414–1423 (2011).
Yu, X., Park, B.H., Wang, M.Y., Wang, Z.V. & Unger, R.H. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc. Natl. Acad. Sci. USA 105, 14070–14075 (2008).
Lee, Y., Wang, M.Y., Du, X.Q., Charron, M.J. & Unger, R.H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60, 391–397 (2011).
Cummings, B.P. et al. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc. Natl. Acad. Sci. USA 108, 14670–14675 (2011).
Maruyama, H., Hisatomi, A., Orci, L., Grodsky, G.M. & Unger, R.H. Insulin within islets is a physiologic glucagon release inhibitor. J. Clin. Invest. 74, 2296–2299 (1984).
Thu, Y.M. & Richmond, A. NF-κB inducing kinase: a key regulator in the immune system and in cancer. Cytokine Growth Factor Rev. 21, 213–226 (2010).
Sun, S.C. Non-canonical NF-κB signaling pathway. Cell Res. 21, 71–85 (2011).
Yin, L. et al. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291, 2162–2165 (2001).
Murray, S.E. et al. NF-κB–inducing kinase plays an essential T cell–intrinsic role in graft-versus-host disease and lethal autoimmunity in mice. J. Clin. Invest. 121, 4775–4786 (2011).
Ling, L., Cao, Z. & Goeddel, D.V. NF-κB–inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc. Natl. Acad. Sci. USA 95, 3792–3797 (1998).
Xiao, G., Fong, A. & Sun, S.C. Induction of p100 processing by NF-κB–inducing kinase involves docking IκB kinase α (IKKα) to p100 and IKKα-mediated phosphorylation. J. Biol. Chem. 279, 30099–30105 (2004).
Xiao, G., Harhaj, E.W. & Sun, S.C. NF-κB–inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 7, 401–409 (2001).
Malinin, N.L., Boldin, M.P., Kovalenko, A.V. & Wallach, D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385, 540–544 (1997).
Ninomiya-Tsuji, J. et al. The kinase TAK1 can activate the NIK-I κB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252–256 (1999).
Sasaki, Y. et al. NIK overexpression amplifies, whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R–mediated survival signals in B cells. Proc. Natl. Acad. Sci. USA 105, 10883–10888 (2008).
Herzig, S. et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183 (2001).
Zhou, X.Y. et al. Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat. Med. 10, 633–637 (2004).
Koo, S.H. et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 (2005).
He, L. et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 (2009).
Yoon, J.C. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001).
Liao, G., Zhang, M., Harhaj, E.W. & Sun, S.C. Regulation of the NF-κB–inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J. Biol. Chem. 279, 26243–26250 (2004).
Vallabhapurapu, S. et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-κB signaling. Nat. Immunol. 9, 1364–1370 (2008).
Zarnegar, B.J. et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9, 1371–1378 (2008).
Duan, C., Yang, H., White, M.F. & Rui, L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol. Cell. Biol. 24, 7435–7443 (2004).
Aoki, K. et al. Dehydroepiandrosterone suppresses the elevated hepatic glucose-6-phosphatase and fructose-1,6-bisphosphatase activities in C57BL/Ksj-db/db mice: comparison with troglitazone. Diabetes 48, 1579–1585 (1999).
Ren, D. et al. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J. Clin. Invest. 117, 397–406 (2007).
Zhou, Y., Jiang, L. & Rui, L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J. Biol. Chem. 284, 11152–11159 (2009).
Huang, W., Dedousis, N., Bhatt, B.A. & O'Doherty, R.M. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J. Biol. Chem. 279, 21695–21700 (2004).
Cho, K.W., Zhou, Y., Sheng, L. & Rui, L. Lipocalin-13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. Mol. Cell. Biol. 31, 450–457 (2011).
Sheng, L., Cho, K.W., Zhou, Y., Shen, H. & Rui, L. Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid β-oxidation. J. Biol. Chem. 286, 38128–38135 (2011).
Acknowledgements
We thank H. Su, Z. Li, C. Duan, D. Morris and S. Wang for assistance and discussion. We thank R. Schreiber (Washington University School of Medicine, St. Louis, Missouri) for providing NIK knockout mice and K. Rajewsky (Immune Disease Institute, Harvard Medical School, Boston, Massachusetts) for providing STOP-NIK mice. Generation of the NIK knockout mice was supported by Amgen Inc., Thousand Oaks, California. This study was supported by grants DK 065122 and DK073601 from the US National Institutes of Health (NIH) and by research award 1-09-RA-156 from the American Diabetes Association. This work used the cores supported by the Michigan Diabetes Research and Training Center (funded by NIH 5P60 DK20572), the University of Michigan's Cancer Center (funded by NIH 5 P30 CA46592), the University of Michigan Nathan Shock Center (funded by NIH P30AG013283) and the University of Michigan Gut Peptide Research Center (funded by NIH DK34933).
Author information
Authors and Affiliations
Contributions
L.R. and L.S. designed the experiments and prepared the manuscript. L.S., Y.Z., Z.C., D.R., K.W.C., L.J. and H.S. performed experiments. Y.S. generated the STOP-NIK mice.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 343 kb)
Rights and permissions
About this article
Cite this article
Sheng, L., Zhou, Y., Chen, Z. et al. NF-κB–inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat Med 18, 943–949 (2012). https://doi.org/10.1038/nm.2756
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2756
This article is cited by
-
NF-κB-Inducing Kinase Provokes Insulin Resistance in Skeletal Muscle of Obese Mice
Inflammation (2023)
-
Biliary NIK promotes ductular reaction and liver injury and fibrosis in mice
Nature Communications (2022)
-
NF-κB-inducing kinase (NIK) is activated in pancreatic β-cells but does not contribute to the development of diabetes
Cell Death & Disease (2022)
-
Hepatic neddylation deficiency triggers fatal liver injury via inducing NF-κB-inducing kinase in mice
Nature Communications (2022)
-
Sam68 promotes hepatic gluconeogenesis via CRTC2
Nature Communications (2021)