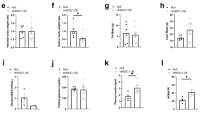
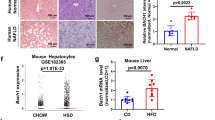
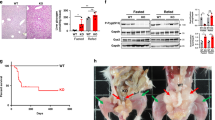
Abstract
Considerable data support the idea that forkhead box O1 (Foxo1) drives the liver transcriptional program during fasting and is then inhibited by thymoma viral proto-oncogene 1 (Akt) after feeding. Here we show that mice with hepatic deletion of Akt1 and Akt2 were glucose intolerant, insulin resistant and defective in their transcriptional response to feeding in the liver. These defects were normalized with concomitant liver-specific deletion of Foxo1. Notably, in the absence of both Akt and Foxo1, mice adapted appropriately to both the fasted and fed state, and insulin suppressed hepatic glucose production normally. A gene expression analysis revealed that deletion of Akt in liver led to the constitutive activation of Foxo1-dependent gene expression, but again, concomitant ablation of Foxo1 restored postprandial regulation, preventing the inhibition of the metabolic response to nutrient intake caused by deletion of Akt. These results are inconsistent with the canonical model of hepatic metabolism in which Akt is an obligate intermediate for proper insulin signaling. Rather, they show that a major role of hepatic Akt is to restrain the activity of Foxo1 and that in the absence of Foxo1, Akt is largely dispensable for insulin- and nutrient-mediated hepatic metabolic regulation in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Saltiel, A.R. & Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
White, M.F. Insulin signaling in health and disease. Science 302, 1710–1711 (2003).
Brown, M.S. & Goldstein, J.L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β). Science 292, 1728–1731 (2001).
Michael, M.D. et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 (2000).
Dong, X.C. et al. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 8, 65–76 (2008).
Chen, W.S. et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203–2208 (2001).
Jacinto, E. et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137 (2006).
Rintelen, F., Stocker, H., Thomas, G. & Hafen, E. PDK1 regulates growth through Akt and S6K in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15020–15025 (2001).
Alessi, D.R. et al. Characterization of a 3-phosphoinositide–dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7, 261–269 (1997).
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 (2002).
Sengupta, S., Peterson, T.R. & Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40, 310–322 (2010).
Düvel, K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 (2010).
Porstmann, T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008).
Laplante, M. & Sabatini, D.M. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc. Natl. Acad. Sci. USA 107, 3281–3282 (2010).
Li, S., Brown, M.S. & Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 107, 3441–3446 (2010).
Chakrabarti, P., English, T., Shi, J., Smas, C.M. & Kandror, K.V. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 59, 775–781 (2010).
Kim, K.H. et al. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1c. J. Biol. Chem. 279, 51999–52006 (2004).
Rena, G., Guo, S., Cichy, S.C., Unterman, T.G. & Cohen, P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274, 17179–17183 (1999).
Nakae, J., Park, B.C. & Accili, D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J. Biol. Chem. 274, 15982–15985 (1999).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Cheng, Z. & White, M.F. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid. Redox Signal. 14, 649–661 (2011).
Puigserver, P. et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423, 550–555 (2003).
Liu, Y. et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 (2008).
Accili, D. & Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426 (2004).
Zhang, W. et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 281, 10105–10117 (2006).
Matsumoto, M., Han, S., Kitamura, T. & Accili, D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 116, 2464–2472 (2006).
O'Brien, R.M., Streeper, R.S., Ayala, J.E., Stadelmaier, B.T. & Hornbuckle, L.A. Insulin-regulated gene expression. Biochem. Soc. Trans. 29, 552–558 (2001).
Biggs, W.H. III, Meisenhelder, J., Hunter, T., Cavenee, W.K. & Arden, K.C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96, 7421–7426 (1999).
Matsumoto, M., Pocai, A., Rossetti, L., Depinho, R.A. & Accili, D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 6, 208–216 (2007).
Haeusler, R.A., Kaestner, K.H. & Accili, D. FoxOs function synergistically to promote glucose production. J. Biol. Chem. 285, 35245–35248 (2010).
Altomonte, J. et al. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am. J. Physiol. Endocrinol. Metab. 285, E718–E728 (2003).
Samuel, V.T. et al. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 55, 2042–2050 (2006).
Easton, R.M. et al. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol. Cell. Biol. 25, 1869–1878 (2005).
Zhang, L., Rubins, N.E., Ahima, R.S., Greenbaum, L.E. & Kaestner, K.H. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2, 141–148 (2005).
Sakoda, H. et al. Differing roles of Akt and serum- and glucocorticoid-regulated kinase in glucose metabolism, DNA synthesis, and oncogenic activity. J. Biol. Chem. 278, 25802–25807 (2003).
Daitoku, H., Yamagata, K., Matsuzaki, H., Hatta, M. & Fukamizu, A. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52, 642–649 (2003).
Perrot, V. & Rechler, M.M. The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol. Endocrinol. 19, 2283–2298 (2005).
van der Vos, K.E. & Coffer, P.J. The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 14, 579–592 (2011).
Hirota, K. et al. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. J. Biol. Chem. 283, 32432–32441 (2008).
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 (2002).
Kovacina, K.S. et al. Identification of a proline-rich Akt substrate as a 14–3-3 binding partner. J. Biol. Chem. 278, 10189–10194 (2003).
Taniguchi, C.M., Emanuelli, B. & Kahn, C.R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006).
Leavens, K.F., Easton, R.M., Shulman, G.I., Previs, S.F. & Birnbaum, M.J. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 10, 405–418 (2009).
Slack, C., Giannakou, M.E., Foley, A., Goss, M. & Partridge, L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell 10, 735–748 (2011).
van der Horst, A. & Burgering, B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 8, 440–450 (2007).
Partridge, L. & Bruning, J.C. Forkhead transcription factors and ageing. Oncogene 27, 2351–2363 (2008).
Yamagata, K. et al. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol. Cell 32, 221–231 (2008).
Housley, M.P. et al. O-GlcNAc regulates FoxO activation in response to glucose. J. Biol. Chem. 283, 16283–16292 (2008).
Huang, H. et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl. Acad. Sci. USA 102, 1649–1654 (2005).
van der Vos, K.E. & Coffer, P.J. FOXO-binding partners: it takes two to tango. Oncogene 27, 2289–2299 (2008).
Okamoto, H., Obici, S., Accili, D. & Rossetti, L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J. Clin. Invest. 115, 1314–1322 (2005).
Gelling, R.W. et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 3, 67–73 (2006).
Obici, S., Zhang, B.B., Karkanias, G. & Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 8, 1376–1382 (2002).
Hill, J.W. et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 11, 286–297 (2010).
Inoue, H. et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 3, 267–275 (2006).
Zhou, Y. et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat. Med. 17, 356–365 (2011).
Wan, M. et al. Loss of Akt1 in mice increases energy expenditure and protects against diet-induced obesity. Mol. Cell. Biol. 32, 96–106 (2011).
Summers, S.A., Whiteman, E.L., Cho, H., Lipfert, L. & Birnbaum, M.J. Differentiation-dependent suppression of platelet-derived growth factor signaling in cultured adipocytes. J. Biol. Chem. 274, 23858–23867 (1999).
Miller, R.A. et al. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J. Clin. Invest. 121, 2518–2528 (2011).
Acknowledgements
The authors would like to thank M. Magnuson (Vanderbilt University) for providing the antibody to Gck and D. Accili (Columbia University) for sharing the Foxo1loxP/loxP mice. We are grateful to J. Schug, who helped with the microarray data analysis and generated the heat map and the density plot. The Functional Genomics Core and the Transgenic, Knockout, Mouse Phenotyping and Biomarker Cores of the University of Pennsylvania Diabetes and Endocrinology Research Center (NIH grant P30 DK19525) were instrumental in this work. This work was supported by the US National Institutes of Health grant RO1 DK56886 (M.J.B.) and the diabetes training grant T32 DK007314 (M.L.).
Author information
Authors and Affiliations
Contributions
M.L. conceived of the hypothesis, designed and performed the experiments and analyzed the data. M.W. and K.F.L. performed experiments. Q.C., B.R.M. and S.F. provided technical assistance. The R.S.A. lab performed the hyperinsulinemic-euglycemic clamps experiments. K.U. and C.R.K. generated the Akt1loxP/loxP mice. M.J.B. conceived the hypothesis and directed the project. M.L. and M.J.B. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 3499 kb)
Supplementary Table 1
Metabolic responsive genes in the control livers (XLS 423 kb)
Supplementary Table 2
Metabolic responsive genes that respond to the loss of Akt in the fed livers (XLS 220 kb)
Rights and permissions
About this article
Cite this article
Lu, M., Wan, M., Leavens, K. et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 18, 388–395 (2012). https://doi.org/10.1038/nm.2686
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2686
This article is cited by
-
Proteogenomics of different urothelial bladder cancer stages reveals distinct molecular features for papillary cancer and carcinoma in situ
Nature Communications (2023)
-
Aerobic exercise promotes the expression of ATGL and attenuates inflammation to improve hepatic steatosis via lncRNA SRA
Scientific Reports (2022)
-
The dynamic clustering of insulin receptor underlies its signaling and is disrupted in insulin resistance
Nature Communications (2022)
-
Transcriptional regulation of N6-methyladenosine orchestrates sex-dimorphic metabolic traits
Nature Metabolism (2021)
-
The aetiology and molecular landscape of insulin resistance
Nature Reviews Molecular Cell Biology (2021)