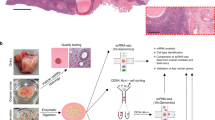
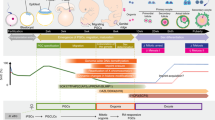
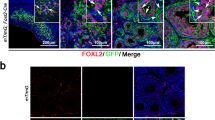
Abstract
Germline stem cells that produce oocytes in vitro and fertilization-competent eggs in vivo have been identified in and isolated from adult mouse ovaries. Here we describe and validate a fluorescence-activated cell sorting-based protocol that can be used with adult mouse ovaries and human ovarian cortical tissue to purify rare mitotically active cells that have a gene expression profile that is consistent with primitive germ cells. Once established in vitro, these cells can be expanded for months and can spontaneously generate 35- to 50-μm oocytes, as determined by morphology, gene expression and haploid (1n) status. Injection of the human germline cells, engineered to stably express GFP, into human ovarian cortical biopsies leads to formation of follicles containing GFP-positive oocytes 1–2 weeks after xenotransplantation into immunodeficient female mice. Thus, ovaries of reproductive-age women, similar to adult mice, possess rare mitotically active germ cells that can be propagated in vitro as well as generate oocytes in vitro and in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zuckerman, S. The number of oocytes in the mature ovary. Recent Prog. Horm. Res. 6, 63–108 (1951).
Johnson, J., Canning, J., Kaneko, T., Pru, J.K. & Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428, 145–150 (2004).
Brinster, R.L. Male germline stem cells: from mice to men. Science 316, 404–405 (2007).
Tilly, J.L., Niikura, Y. & Rueda, B.R. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol. Reprod. 80, 2–12 (2009).
Zou, K. et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 11, 631–636 (2009).
Pacchiarotti, J. et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 79, 159–170 (2010).
Johnson, J. et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 122, 303–315 (2005).
Wang, N. & Tilly, J.L. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle 9, 339–349 (2010).
Niikura, Y., Niikura, T., Wang, N., Satirapod, C. & Tilly, J.L. Systemic signals in aged males exert potent rejuvenating effects on the ovarian follicle reserve in mammalian females. Aging 2, 999–1003 (2010).
Tilly, J.L. & Telfer, E.E. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol. Hum. Reprod. 15, 393–398 (2009).
Niikura, Y., Niikura, T. & Tilly, J.L. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging 1, 971–978 (2009).
Massasa, E., Costa, X.S. & Taylor, H.S. Failure of the stem cell niche rather than loss of oocyte stem cells in the aging ovary. Aging 2, 1–2 (2010).
Fujiwara, Y. et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc. Natl. Acad. Sci. USA 91, 12258–12262 (1994).
Castrillon, D.H., Quade, B.J., Wang, T.Y., Quigley, C. & Crum, C.P. The human VASA gene is specifically expressed in the germ lineage. Proc. Natl. Acad. Sci. USA 97, 9585–9590 (2000).
Noce, T., Okamoto-Ito, S. & Tsunekawa, N. Vasa homolog genes in mammalian germ cell development. Cell Struct. Funct. 26, 131–136 (2001).
Normile, D. Reproductive biology. Study suggests a renewable source of eggs and stirs more controversy. Science 324, 320 (2009).
Saitou, M., Barton, S.C. & Surani, M.A. A molecular programme for the specification of germ cell fate in mice. Nature 418, 293–300 (2002).
Ohinata, Y. et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436, 207–213 (2005).
Dolci, S. et al. Stem cell factor activates telomerase in mouse mitotic spermatogonia and in primordial germ cells. J. Cell Sci. 115, 1643–1649 (2002).
Gong, S.P. et al. Embryonic stem cell-like cells established by culture of adult ovarian cells in mice. Fertil. Steril. 93, 2594–2601 (2010).
Zou, K., Hou, L., Sun, K., Xie, W. & Wu, J. Improved efficiency of female germline stem cell purification using Fragilis-based magnetic bead sorting. Stem Cells Dev. 20, 2197–2204 (2011).
Suzumori, N., Yan, C., Matzuk, M.M. & Rajkovic, A. Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech. Dev. 111, 137–141 (2002).
Rajkovic, A., Pangas, S.A., Ballow, D., Suzumori, N. & Matzuk, M.M. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 305, 1157–1159 (2004).
Pangas, S.A. et al. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc. Natl. Acad. Sci. USA 103, 8090–8095 (2006).
Elvin, J.A., Clark, A.T., Wang, P., Wolfman, N.M. & Matzuk, M.M. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol. 13, 1035–1048 (1999).
Zheng, P. & Dean, J. Oocyte-specific genes affect folliculogenesis, fertilization, and early development. Semin. Reprod. Med. 25, 243–251 (2007).
Gu, W. et al. Mammalian male and female germ cells express a germ cell-specific Y-Box protein, MSY2. Biol. Reprod. 59, 1266–1274 (1998).
Yang, J. et al. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc. Natl. Acad. Sci. USA 102, 5755–5760 (2005).
Page, S.L. & Hawley, R.S. The genetics and molecular biology of the synaptonemal complex. Annu. Rev. Cell Dev. Biol. 20, 525–558 (2004).
Yuan, L. et al. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science 296, 1115–1118 (2002).
Kagawa, W. & Kurumizaka, H. From meiosis to postmeiotic events: uncovering the molecular roles of the meiosis-specific recombinase Dmc1. FEBS J. 277, 590–598 (2010).
West, F.D., Mumaw, J.L., Gallegos-Cardenas, A., Young, A. & Stice, S.L. Human haploid cells differentiated from meiotic competent clonal germ cell lines that originated from embryonic stem cells. Stem Cells Dev. 20, 1079–1088 (2011).
Abban, G. & Johnson, J. Stem cell support of oogenesis in the human. Hum. Reprod. 24, 2974–2978 (2009).
Brinster, R.L. & Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 91, 11298–11302 (1994).
Brinster, C.J. et al. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol. Reprod. 69, 412–420 (2003).
Oktay, K. & Karlikaya, G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N. Engl. J. Med. 342, 1919 (2000).
Sönmezer, M. & Oktay, K. Orthotopic and heterotopic ovarian tissue transplantation. Best Pract. Res. Clin. Obstet. Gynaecol. 24, 113–126 (2010).
Hübner, K. et al. Derivation of oocytes from mouse embryonic stem cells. Science 300, 1251–1256 (2003).
Ko, K. & Schöler, H.R. Embryonic stem cells as a potential source of gametes. Semin. Reprod. Med. 24, 322–329 (2006).
Nicholas, C.R., Haston, K.M., Grewall, A.K., Longacre, T.A. & Reijo Pera, R.A. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum. Mol. Genet. 18, 4376–4389 (2009).
Kee, K., Angeles, V.T., Flores, M., Nguyen, H.N. & Reijo Pera, R.A. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 462, 222–225 (2009).
Nicholas, C.R., Chavez, S.L., Baker, V.L. & Reijo Pera, R.A. Instructing an embryonic stem cell-derived oocyte fate: lessons from endogenous oogenesis. Endocr. Rev. 30, 264–283 (2009).
Yeom, Y.I. et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881–894 (1996).
Yoshimizu, T. et al. Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev. Growth Differ. 41, 675–684 (1999).
Szabó, P.E. et al. Allele specific expression of imprinted genes in mouse migratory primordial germ cells. Mech. Dev. 115, 157–160 (2002).
Lee, H.-J. et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J. Clin. Oncol. 25, 3198–3204 (2007).
Kagawa, N., Silber, S. & Kuwayama, M. Successful vitrification of bovine and human ovarian tissue. Reprod. Biomed. Online 18, 568–577 (2009).
Selesniemi, K., Lee, H.-J., Muhlhauser, A. & Tilly, J.L. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. USA 108, 12319–12324 (2011).
Weissman, A. et al. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol. Reprod. 60, 1462–1467 (1999).
Matikainen, T. et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 28, 355–360 (2001).
Acknowledgements
The authors thank L. Prickett-Rice and K. Folz-Donahue of the Harvard Stem Cell Institute Flow Cytometry Core Facility and J. Groeneweg for expert technical assistance. We also thank J.R. Mann and K.J. MacLaughlin for the provision of TgOG2 transgenic mice. This work was supported by a Method to Extend Research in Time (MERIT) Award from the US National Institute on Aging (NIH R37-AG012279), the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation and Vincent Memorial Hospital Research Funds. This work was conducted while D.C.W. was supported in part by a Ruth L. Kirschstein National Research Service Award (NIH F32-AG034809).
Author information
Authors and Affiliations
Contributions
Y.A.R.W., D.C.W. and J.L.T. designed the experiments, analyzed the data and wrote the manuscript. Y.A.R.W. and D.C.W. conducted the experiments. Y.T., O.I. and H.S. collected, cryopreserved and provided human ovarian cortical tissue. J.L.T. directed the project. All authors reviewed and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
J.L.T. declares interest in intellectual property described in US Patent 7,955,846 and is a co-founder of OvaScience, Inc., and Y.A.R.W. and D.C.W. are scientific consultants for OvaScience, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Tables 1 and 2 and Supplementary Figures 1–5 (PDF 1403 kb)
Rights and permissions
About this article
Cite this article
White, Y., Woods, D., Takai, Y. et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med 18, 413–421 (2012). https://doi.org/10.1038/nm.2669
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2669
This article is cited by
-
Discussion of field effects after intraovarian injection of autologous platelet-rich plasma
Bulletin of the National Research Centre (2023)
-
In-vivo oogenesis of oogonial and mesenchymal stem cells seeded in transplanted ovarian extracellular matrix
Journal of Ovarian Research (2023)
-
Metformin Promotes Proliferation of Mouse Female Germline Stem Cells by Histone Acetylation Modification of Traf2
Stem Cell Reviews and Reports (2023)
-
Meiosis resumption in human primordial germ cells from induced pluripotent stem cells by in vitro activation and reconstruction of ovarian nests
Stem Cell Research & Therapy (2022)
-
The Future of IVF: The New Normal in Human Reproduction
Reproductive Sciences (2022)