Abstract
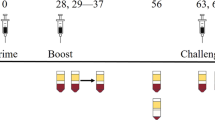
Vaccine-induced immunity to Ebola virus infection in nonhuman primates (NHPs) is marked by potent antigen-specific cellular and humoral immune responses1,2; however, the immune mechanism of protection remains unknown. Here we define the immune basis of protection conferred by a highly protective recombinant adenovirus virus serotype 5 (rAd5) encoding Ebola virus glycoprotein (GP)1,3 in NHPs. Passive transfer of high-titer polyclonal antibodies from vaccinated Ebola virus–immune cynomolgus macaques to naive macaques failed to confer protection against disease, suggesting a limited role of humoral immunity. In contrast, depletion of CD3+ T cells in vivo after vaccination and immediately before challenge eliminated immunity in two vaccinated macaques, indicating a crucial requirement for T cells in this setting. The protective effect was mediated largely by CD8+ cells, as depletion of CD8+ cells in vivo using the cM-T807 monoclonal antibody (mAb), which does not affect CD4+ T cell or humoral immune responses, abrogated protection in four out of five subjects. These findings indicate that CD8+ cells have a major role in rAd5-GP–induced immune protection against Ebola virus infection in NHPs. Understanding the immunologic mechanism of Ebola virus protection will facilitate the development of vaccines for Ebola and related hemorrhagic fever viruses in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sullivan, N.J. et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424, 681–684 (2003).
Sullivan, N.J., Sanchez, A., Rollin, P.E., Yang, Z.Y. & Nabel, G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408, 605–609 (2000).
Sullivan, N.J. et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 3, e177 (2006).
Jones, S.M. et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11, 786–790 (2005).
Geisbert, T.W. et al. Vector choice determines immunogenicity and potency of genetic vaccines against Angola Marburg virus in nonhuman primates. J. Virol. 84, 10386–10394 (2010).
Sullivan, N.J., Martin, J.E., Graham, B.S. & Nabel, G.J. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 7, 393–400 (2009).
Oswald, W.B. et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog. 3, e9 (2007).
Neville, D.M. Jr. et al. A new reagent for the induction of T cell depletion, anti-CD3–CRM9. J. Immunother. Emphasis Tumor Immunol. 19, 85–92 (1996).
Hensley, L.E. et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus species. PLoS Pathog. 6, e1000904 (2010).
Schmitz, J.E. et al. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am. J. Pathol. 154, 1923–1932 (1999).
Gupta, M. et al. Persistent infection with Ebola virus under conditions of partial immunity. J. Virol. 78, 958–967 (2004).
Wilson, J.A. & Hart, M.K. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J. Virol. 75, 2660–2664 (2001).
Warfield, K.L. et al. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J. Immunol. 175, 1184–1191 (2005).
Jahrling, P.B. et al. Passive immunization of Ebola virus–infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch. Virol. Suppl. 11, 135–140 (1996).
Geisbert, T.W. et al. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg. Infect. Dis. 8, 503–507 (2002).
Geisbert, T.W. et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163, 2347–2370 (2003).
Sanchez, A., Kiley, M.P., Holloway, B.P. & Auperin, D.D. Sequence analysis of the Ebola virus genome: organization, genetic elements and comparison with the genome of Marburg virus. Virus Res. 29, 215–240 (1993).
Xu, L. et al. Immunization for Ebola virus infection. Nat. Med. 4, 37–42 (1998).
Ohno, T. et al. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science 265, 781–784 (1994).
Chattopadhyay, P.K, Yu, J. & Roederer, M. Application of quantum dots to multicolor flow cytometry. in Methods in Molecular Biology (eds. Bruchez, M. & Hotz, C.) 374, 175–184 (Humana Press, Totowa, New Jersey, 2007).
Geisbert, T.W. et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet 375, 1896–1905 (2010).
Acknowledgements
We thank D. Jeffers, T. Suhana, A. Tislerics and B. Hartman for help with manuscript preparation; M. Cichanowski for graphics; D. Braun, K. Daddario and C. Rice for technical and animal care assistance; S. Norris and M. Nason for assistance with statistical considerations; and K. Kenyon for editorial support. We thank D. Neville (US National Institute of Mental Health) for the generous gift of FN18-CRM9 immunotoxin. Reagent support was provided by the Nonhuman Primate Reagent Resource (grant number R24RR016001 from the National Center for Research Resource, US National Institutes of Health). Support for this work was provided by the Intramural Research Program of the US National Institutes of Health. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the US Army or the Department of Defense.
Author information
Authors and Affiliations
Contributions
N.J.S. and G.J.N. conceived of the studies and wrote the manuscript. T.W.G., J.B.G., L.H., J.J., G.O. and A.H. contributed to animal study design, conducted infectious Ebola virus challenge, post-challenge assays and passive antibody administration. N.J.S., M.B., C.A. and D.S. performed vaccine preparation, animal immunization, characterization of vaccine-induced immune responses and immunodepleting antibody administration. K.A.R. provided depleting antibodies and contributed to experimental design. S.B. and S.R. performed immunohistochemistry. M.R., P.B.J. and R.A.K. contributed to experimental design or provided reagents.
Corresponding author
Ethics declarations
Competing interests
N.J.S., G.J.N., T.W.G. and P.B.J. have intellectual property on gene-based vaccines for Ebola.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 1170 kb)
Rights and permissions
About this article
Cite this article
Sullivan, N., Hensley, L., Asiedu, C. et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med 17, 1128–1131 (2011). https://doi.org/10.1038/nm.2447
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2447
This article is cited by
-
The co-delivery of adenovirus-based immune checkpoint vaccine elicits a potent anti-tumor effect in renal carcinoma
npj Vaccines (2023)
-
Characterisation of the T-cell response to Ebola virus glycoprotein amongst survivors of the 2013–16 West Africa epidemic
Nature Communications (2021)
-
Characterization of 100 extended major histocompatibility complex haplotypes in Indonesian cynomolgus macaques
Immunogenetics (2020)
-
Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate
npj Vaccines (2020)
-
An adenovirus serotype 2-vectored ebolavirus vaccine generates robust antibody and cell-mediated immune responses in mice and rhesus macaques
Emerging Microbes & Infections (2018)