Abstract
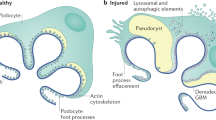
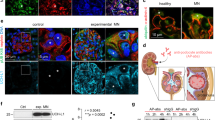
Focal segmental glomerulosclerosis (FSGS) is a cause of proteinuric kidney disease, compromising both native and transplanted kidneys. Treatment is limited because of a complex pathogenesis, including unknown serum factors. Here we report that serum soluble urokinase receptor (suPAR) is elevated in two-thirds of subjects with primary FSGS, but not in people with other glomerular diseases. We further find that a higher concentration of suPAR before transplantation underlies an increased risk for recurrence of FSGS after transplantation. Using three mouse models, we explore the effects of suPAR on kidney function and morphology. We show that circulating suPAR activates podocyte β3 integrin in both native and grafted kidneys, causing foot process effacement, proteinuria and FSGS-like glomerulopathy. Our findings suggest that the renal disease only develops when suPAR sufficiently activates podocyte β3 integrin. Thus, the disease can be abrogated by lowering serum suPAR concentrations through plasmapheresis, or by interfering with the suPAR–β3 integrin interaction through antibodies and small molecules targeting either uPAR or β3 integrin. Our study identifies serum suPAR as a circulating factor that may cause FSGS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Kitiyakara, C., Eggers, P. & Kopp, J.B. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am. J. Kidney Dis. 44, 815–825 (2004).
Baum, M.A. Outcomes after renal transplantation for FSGS in children. Pediatr. Transplant. 8, 329–333 (2004).
Senggutuvan, P. et al. Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: analysis of incidence and risk factors in 59 allografts. Pediatr. Nephrol. 4, 21–28 (1990).
Hickson, L.J. et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation 87, 1232–1239 (2009).
Ponticelli, C. & Glassock, R.J. Post-transplant recurrence of primary glomerulonephritis. Clin. J. Am. Soc. Nephrol. 5, 2363–2372 (2010).
Tryggvason, K., Patrakka, J. & Wartiovaara, J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N. Engl. J. Med. 354, 1387–1401 (2006).
Mathieson, P.W. Proteinuria and immunity—an overstated relationship? N. Engl. J. Med. 359, 2492–2494 (2008).
Savin, V.J. et al. Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Transl. Res. 151, 288–292 (2008).
Hoyer, J.R. et al. Recurrence of idiopathic nephrotic syndrome after renal transplantation. J. Am. Soc. Nephrol. 12, 1994–2002 (2001).
Artero, M.L. et al. Plasmapheresis reduces proteinuria and serum capacity to injure glomeruli in patients with recurrent focal glomerulosclerosis. Am. J. Kidney Dis. 23, 574–581 (1994).
Haas, M. et al. Plasma immunadsorption treatment in patients with primary focal and segmental glomerulosclerosis. Nephrol. Dial. Transplant. 13, 2013–2016 (1998).
Kemper, M.J., Wolf, G. & Muller-Wiefel, D.E. Transmission of glomerular permeability factor from a mother to her child. N. Engl. J. Med. 344, 386–387 (2001).
Glassock, R.J. Circulating permeability factors in the nephrotic syndrome: A fresh look at an old problem. J. Am. Soc. Nephrol. 14, 541–543 (2003).
Savin, V.J. et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N. Engl. J. Med. 334, 878–883 (1996).
Sharma, M., Sharma, R., McCarthy, E.T. & Savin, V.J. “The FSGS factor”: enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J. Am. Soc. Nephrol. 10, 552–561 (1999).
Bruneau, S. et al. Potential role of soluble ST2 protein in idiopathic nephrotic syndrome recurrence following kidney transplantation. Am. J. Kidney Dis. 54, 522–532 (2009).
McCarthy, E.T., Sharma, M. & Savin, V.J. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 5, 2115–2121 (2010).
Wei, C. et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 14, 55–63 (2008).
Blasi, F. & Carmeliet, P. uPAR: A versatile signaling orchestrator. Nat. Rev. Mol. Cell Biol. 3, 932–943 (2002).
Smith, H.W. & Marshall, C.J. Regulation of cell signaling by uPAR. Nat. Rev. Mol. Cell Biol. 11, 23–36 (2010).
Sier, C.F. et al. The level of urokinase-type plasminogen activator receptor is increased in the serum of ovarian cancer patients. Cancer Res. 58, 1843–1849 (1998).
Sidenius, N. et al. Serum level of soluble urokinase-type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood 96, 4091–4095 (2000).
Young, B.C., Levine, R.J. & Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. 5, 173–192 (2010).
Ploug, M. & Ellis, V. Structure-function relationship in the receptor for urokinase-type plasminogen activator. Comparison to other members of the Ly-6 family and snake venom a-neurotoxins. FEBS Lett. 349, 163–168 (1994).
Kristensen, P., Eriksen, J., Blasi, F. & Dano, K. Two alternatively spliced mouse urokinase receptor mRNAs with different histological localization in the gastrointestinal tract. J. Cell Biol. 115, 1763–1771 (1991).
Hada, Y. et al. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem. Biophys. Res. Commun. 356, 487–493 (2007).
D'Alessio, S. & Blasi, F. The urokinase receptor as an entertainer of signal transduction. Front. Biosci. 14, 4575–4587 (2009).
Selleri, C. et al. In vivo activity of the cleaved form of soluble urokinase receptor: a new hematopoietic stem/progenitor cell mobilizer. Cancer Res. 66, 10885–10890 (2006).
Pampori, N. et al. Mechanisms and consequences of affinity modulation of integrin αVβ3 detected with a novel patch-engineered monovalent ligand. J. Biol. Chem. 274, 21609–21616 (1999).
Honda, S. et al. Topography of ligand-induced binding sites, including a novel cation-sensitive epitope (AP5) at the amino terminus, of the human integrin beta 3 subunit. J. Biol. Chem. 270, 11947–11954 (1995).
Saleem, M.A. et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 (2002).
Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003).
Mundel, P. et al. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol. 139, 193–204 (1997).
Ghiggeri, G.M., Carraro, M. & Vincenti, F. Recurrent focal segmental glomerulosclerosis in the era of genetics of podocyte proteins: theory and therapy. Nephrol. Dial. Transplant. 19, 1036–1040 (2004).
Dekkers, P.E., ten Hove, T., te Velde, A.A., van Deventer, S.J. & van Der Poll, T. Upregulation of monocyte urokinase plasminogen activator receptor during human endotoxemia. Infect. Immun. 68, 2156–2160 (2000).
Crowley, S.D. et al. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J. Clin. Invest. 119, 943–953 (2009).
Hoyer, J.R. et al. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet 2, 343–348 (1972).
Gohh, R.Y. et al. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am. J. Transplant. 5, 2907–2912 (2005).
Beck, L.H. Jr. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 36, 11–21 (2009).
Clement, L.C. et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 17, 117–122 (2011).
Zhang, S.Y. et al. c-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci. Signal. 18, ra39 (2010).
Einecke, G. et al. A molecular classifier for predicting future graft loss in late kidney transplant biopsies. J. Clin. Invest. 120, 1862–1872 (2010).
Vijayan, K.V., Goldschmidt-Clermont, P.J., Roos, C. & Bray, P.F. The PIA2 polymorphism of integrin β3 enhances outside-in signaling and adhesive functions. J. Clin. Invest. 105, 793–802 (2000).
Salido, E. et al. The polymorphism of the platelet glycoprotein IIIA gene as a risk factor for acute renal allograft rejection. J. Am. Soc. Nephrol. 10, 2599–2605 (1999).
Chiras, T. et al. Platelet GPIIIa polymorphism HPA-1 (PLA1/2) is associated with hypertension as the primary cause for end-stage renal disease in hemodialysis patients from Greece. In Vivo 23, 177–181 (2009).
Marszal, J. & Saleem, M.A. The bioactivity of plasma factors in focal segmental glomerulosclerosis. Nephron Exp. Nephrol. 104, e1–e5 (2006).
Tjwa, M. et al. Membrane-anchored uPAR regulates the proliferation, marrow pool size, engraftment, and mobilization of mouse hematopoietic stem/progenitor cells. J. Clin. Invest. 119, 1008–1018 (2009).
Acknowledgements
We thank N. Sidenius (Foundation FIRC Institute of Molecular Oncology, Italy) for help with the suPAR assay in mouse samples. We are grateful to L.H. Beck, Jr. and D. Salant (Boston University Medical Center) for providing the membranous nephropathy patient cohort. We thank S. Hsiesh for help with sample collection. We thank G. Høyer-Hansen (the Finsen Laboratory, Denmark) for additional suPAR assays and discussions. The authors are grateful to P.J. Goldschmidt for helpful scientific discussions regarding the manuscript and to M.J. Tracy for critical reading of the manuscript. This work was supported in part by the US National Institutes of Health (grants DK073495 and DK089394 to J.R., DK-82636 to A.F., DK070011 to G.B.), the Halpin Foundation–American Society of Nephrology Research Grant (to C.W.), a grant from the American Diabetes Association (7-09-JF-23 to A.F.), and a grant from the Diabetes Research Institute Foundation (to A.F.). The authors also wish to acknowledge the generous support of the Katz Family Fund.
Author information
Authors and Affiliations
Contributions
J.R. conceived the study. J.R. and C.W. designed the experiments, coordinated the study, analyzed the data and wrote the manuscript. C.W., S.E.H., J.L., D.M., Q.Z., B.N., P.D., V.G. performed the experiments. A.F., N.G., G.B., J.S., S.A.K., H.-K.Y., M.Saleem, A.C., E.S., A.T., M.Salifu, M.M.S., F.S., C.M., V.S., M.Z., D.R., M.P.R., P.R., J.R. contributed to clinical samples and clinical information. M.P.R. and P.R. provided pathology service.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1–3 and Supplementary Methods (PDF 668 kb)
Rights and permissions
About this article
Cite this article
Wei, C., El Hindi, S., Li, J. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17, 952–960 (2011). https://doi.org/10.1038/nm.2411
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2411
This article is cited by
-
Rationale and design of the Japanese Biomarkers in Nephrotic Syndrome (J-MARINE) study
Clinical and Experimental Nephrology (2024)
-
XOR risk variants drive diabetic kidney disease
Nature Metabolism (2023)
-
Repetitive administration of rituximab can achieve and maintain clinical remission in patients with MCD or FSGS
Scientific Reports (2023)
-
What is circulating factor disease and how is it currently explained?
Pediatric Nephrology (2023)
-
SuPAR mediates viral response proteinuria by rapidly changing podocyte function
Nature Communications (2023)