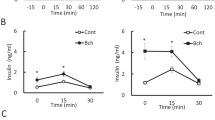
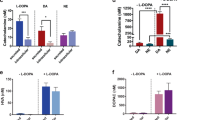
Abstract
Acetylcholine is a neurotransmitter that has a major role in the function of the insulin-secreting pancreatic beta cell1,2. Parasympathetic innervation of the endocrine pancreas, the islets of Langerhans, has been shown to provide cholinergic input to the beta cell in several species1,3,4, but the role of autonomic innervation in human beta cell function is at present unclear. Here we show that, in contrast to the case in mouse islets, cholinergic innervation of human islets is sparse. Instead, we find that the alpha cells of human islets provide paracrine cholinergic input to surrounding endocrine cells. Human alpha cells express the vesicular acetylcholine transporter and release acetylcholine when stimulated with kainate or a lowering in glucose concentration. Acetylcholine secretion by alpha cells in turn sensitizes the beta cell response to increases in glucose concentration. Our results demonstrate that in human islets acetylcholine is a paracrine signal that primes the beta cell to respond optimally to subsequent increases in glucose concentration. Cholinergic signaling within islets represents a potential therapeutic target in diabetes5, highlighting the relevance of this advance to future drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gilon, P. & Henquin, J. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 22, 565–604 (2001).
Gautam, D. et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 3, 449–461 (2006).
Ahrén, B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43, 393–410 (2000).
Conn, P.M., Goodman, H.M. & Kostyo, J.L. The Endocrine System: The Endocrine Pancreas and Regulation of Metabolism. 153 (Oxford University Press, New York, 1998).
Gautam, D. et al. Role of the M3 muscarinic acetylcholine receptor in beta-cell function and glucose homeostasis. Diabetes Obes. Metab. 9 (Suppl 2), 158–169 (2007).
Guenifi, A., Simonsson, E., Karlsson, S., Ahrén, B. & Abdel-Halim, S. Carbachol restores insulin release in diabetic GK rat islets by mechanisms largely involving hydrolysis of diacylglycerol and direct interaction with the exocytotic machinery. Pancreas 22, 164–171 (2001).
Doliba, N.M. et al. Restitution of defective glucose-stimulated insulin release of sulfonylurea type 1 receptor knockout mice by acetylcholine. Am. J. Physiol. Endocrinol. Metab. 286, E834–E843 (2004).
Guo, Y. et al. CHRM3 gene variation is associated with decreased acute insulin secretion and increased risk for early-onset type 2 diabetes in Pima Indians. Diabetes 55, 3625–3629 (2006).
Woods, S.C. & Porte, D.J. Neural control of the endocrine pancreas. Physiol. Rev. 54, 596–619 (1974).
Coupland, R.E. The innervation of pan creas of the rat, cat and rabbit as revealed by the cholinesterase technique. J. Anat. 92, 143–149 (1958).
Amenta, F., Cavallotti, C., de Rossi, M., Tonelli, F. & Vatrella, F. The cholinergic innervation of human pancreatic islets. Acta Histochem. 73, 273–278 (1983).
Ahrén, B., Taborsky, G.J. & Porte, D.J. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 29, 827–836 (1986).
Brunicardi, F.C., Shavelle, D. & Andersen, D. Neural regulation of the endocrine pancreas. Int. J. Pancreatol. 18, 177–195 (1995).
Weihe, E., Tao-Cheng, J., Schäfer, M., Erickson, J. & Eiden, L. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc. Natl. Acad. Sci. USA 93, 3547–3552 (1996).
Karlsson, S., Myrsén, U., Nieuwenhuizen, A., Sundler, F. & Ahrén, B. Presynaptic sympathetic mechanism in the insulinostatic effect of epinephrine in mouse pancreatic islets. Am. J. Physiol. 272, R1371–R1378 (1997).
Cabrera, O. et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 7, 545–554 (2008).
Eiden, L.E. The cholinergic gene locus. J. Neurochem. 70, 2227–2240 (1998).
Barlow, A.L., Macleod, A., Noppen, S., Sanderson, J. & Guérin, C.J. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of pearson's correlation coefficient. Microsc. Microanal. 16, 710–724 (2010).
Liu, Y. & Edwards, R. Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells. J. Cell Biol. 139, 907–916 (1997).
Krantz, D.E. et al. A phosphorylation site regulates sorting of the vesicular acetylcholine transporter to dense core vesicles. J. Cell Biol. 149, 379–396 (2000).
Greider, M.H., Bencosme, S. & Lechago, J. The human pancreatic islet cells and their tumors. I. The normal pancreatic islets. Lab. Invest. 22, 344–354 (1970).
Cabrera, O. et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant. 16, 1039–1048 (2008).
Jacques-Silva, M.C. et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc. Natl. Acad. Sci. USA 107, 6465–6470 (2010).
Zawalich, W.S., Zawalich, K. & Rasmussen, H. Cholinergic agonists prime the beta-cell to glucose stimulation. Endocrinology 125, 2400–2406 (1989).
Cabrera, O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 103, 2334–2339 (2006).
Stagner, J.I., Samols, E. & Weir, G.C. Sustained oscillations of insulin, glucagon, and somatostatin from the isolated canine pancreas during exposure to a constant glucose concentration. J. Clin. Invest. 65, 939–942 (1980).
Lang, D.A., Matthews, D.R., Peto, J. & Turner, R.C. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N. Engl. J. Med. 301, 1023–1027 (1979).
Wessler, I. & Kirkpatrick, C. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 154, 1558–1571 (2008).
Brissova, M. et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53, 1087–1097 (2005).
Bosco, D. et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59, 1202–1210 (2010).
Pisania, A. et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Invest. 90, 1661–1675 (2010).
Stagner, J.I. & Samols, E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41, 93–97 (1992).
Taylor, I.L. & Feldman, M. Effect of cephalic-vagal stimulation on insulin, gastric inhibitory polypeptide, and pancreatic polypeptide release in humans. J. Clin. Endocrinol. Metab. 55, 1114–1117 (1982).
Teff, K.L., Mattes, R., Engelman, K. & Mattern, J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism 42, 1600–1608 (1993).
Becker, H.D., Börger, H. & Schafmayer, A. Effect of vagotomy on gastrointestinal hormones. World J. Surg. 3, 615–622 (1979).
Pozza, G. et al. Metabolic control of type I (insulin dependent) diabetes after pancreas transplantation. Br. Med. J. (Clin. Res. Ed.) 291, 510–513 (1985).
Diem, P. et al. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J. Clin. Invest. 86, 2008–2013 (1990).
Blackman, J.D. et al. Insulin secretory profiles and C-peptide clearance kinetics at 6 months and 2 years after kidney-pancreas transplantation. Diabetes 41, 1346–1354 (1992).
Huang, Y.J. et al. Mouse taste buds use serotonin as a neurotransmitter. J. Neurosci. 25, 843–847 (2005).
Buck, M.A. & Fraser, C. Muscarinic acetylcholine receptor subtypes which selectively couple to phospholipase C: pharmacological and biochemical properties. Biochem. Biophys. Res. Commun. 173, 666–672 (1990).
Acknowledgements
We thank A. Formoso for help with data analyses, K. Johnson for histological work and M. Correa-Medina for assistance with RT-PCR. This work was funded by the Diabetes Research Institute Foundation, US National Institutes of Health grants R56DK084321 (A.C.), R01DK084321 (A.C.), R01DC000374 (S.D.R.), R01DC007630 (S.D.R.), F32DK083226 (M.H.A.) and 5U01DK070460-07 (C.R.), the Juvenile Diabetes Research Foundation, the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Diabetes Association, the Family Erling-Persson Foundation and the World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10105-0). Human islets were made available through the Islet Cell Resource basic science islet distribution program, Islet Cell Resource Centers Consortium, Division of Clinical Research, National Center for Research Resources, US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
R.R.-D., M.C.J.-S., A.F. and J.M. performed hormone assay experiments and ELISAs; R.D. performed experiments with biosensor cells to detect acetylcholine secretion; R.R.-D. and M.C.J.-S. conducted Amplex assays to measure acetylcholine secretion; R.R.-D. and M.H.A. collected, analyzed and quantified immunohistochemical data, and R.R.-D. performed RT-PCR and western blotting. R.R.-D., R.D., M.H.A., C.R., S.D.R., P.-O.B. and A.C. designed the study, analyzed data and wrote the paper. R.R.-D. and R.D. contributed equally to the study. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 662 kb)
Supplementary Video 1
Three-dimensional rendering of the vAChT immunostaining pattern in a mouse islet. Nerve fibers immunostained for vAChT are visualized as they enter the core of the islet to innervate beta cells. Reconstruction based on 45 confocal images (z step = 0.3 µm) was performed using Volocity Visualization software. (AVI 89557 kb)
Supplementary Video 2
Three-dimensional rendering of the vAChT immunostaining pattern in a human islet. Strong immunostaining is present in cells expressing glucagon. Immunostained nerve fibers cannot be seen in the islet but are visible in the exocrine tissue. Reconstruction based on 45 confocal images (z step = 0.3 µm) was performed using Volocity Visualization software. (AVI 99452 kb)
Rights and permissions
About this article
Cite this article
Rodriguez-Diaz, R., Dando, R., Jacques-Silva, M. et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 17, 888–892 (2011). https://doi.org/10.1038/nm.2371
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2371
This article is cited by
-
Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases
Nature Reviews Endocrinology (2023)
-
Pancreatic β-cell heterogeneity in adult human islets and stem cell-derived islets
Cellular and Molecular Life Sciences (2023)
-
Regulation of local alternating electric fields on synaptic plasticity in brain tissue
Biomedical Engineering Letters (2023)
-
Optogenetic stimulation of vagal nerves for enhanced glucose-stimulated insulin secretion and β cell proliferation
Nature Biomedical Engineering (2023)
-
In vivo vesicular acetylcholine transporter density in human peripheral organs: an [18F]FEOBV PET/CT study
EJNMMI Research (2022)