Abstract
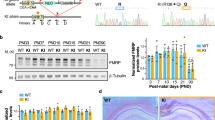
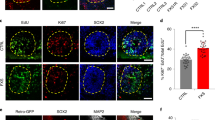
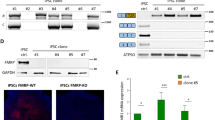
Deficiency in fragile X mental retardation protein (FMRP) results in fragile X syndrome (FXS), an inherited form of intellectual disability. Despite extensive research, it is unclear how FMRP deficiency contributes to the cognitive deficits in FXS. Fmrp-null mice show reduced adult hippocampal neurogenesis. As Fmrp is also enriched in mature neurons, we investigated the function of Fmrp expression in neural stem and progenitor cells (aNSCs) and its role in adult neurogenesis. Here we show that ablation of Fmrp in aNSCs by inducible gene recombination leads to reduced hippocampal neurogenesis in vitro and in vivo, as well as markedly impairing hippocampus-dependent learning in mice. Conversely, restoration of Fmrp expression specifically in aNSCs rescues these learning deficits in Fmrp-deficient mice. These data suggest that defective adult neurogenesis may contribute to the learning impairment seen in FXS, and these learning deficits can be rectified by delayed restoration of Fmrp specifically in aNSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Deng, W., Aimone, J.B. & Gage, F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 (2010).
Kempermann, G., Krebs, J. & Fabel, K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry 21, 290–295 (2008).
Clelland, C.D. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009).
Deng, W., Saxe, M.D., Gallina, I.S. & Gage, F.H. Adult–born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542 (2009).
Dupret, D. et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3, e1959 (2008).
Farioli–Vecchioli, S. et al. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 6, e246 (2008).
Imayoshi, I. et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153–1161 (2008).
Saxe, M.D. et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA 103, 17501–17506 (2006).
Creer, D.J., Romberg, C., Saksida, L.M., van Praag, H. & Bussey, T.J. Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. USA 107, 2367–2372 (2010).
Kitamura, T. et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139, 814–827 (2009).
Penagarikano, O., Mulle, J.G. & Warren, S.T. The pathophysiology of fragile X syndrome. Annu. Rev. Genomics Hum. Genet. 8, 109–129 (2007).
Lightbody, A.A. & Reiss, A.L. Gene, brain, and behavior relationships in fragile X syndrome: evidence from neuroimaging studies. Dev. Disabil. Res. Rev. 15, 343–352 (2009).
Mineur, Y.S., Huynh, L.X. & Crusio, W.E. Social behavior deficits in the Fmr1 mutant mouse. Behav. Brain Res. 168, 172–175 (2006).
Dobkin, C. et al. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience 100, 423–429 (2000).
D'Hooge, R. et al. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience 76, 367–376 (1997).
Brennan, F.X., Albeck, D.S. & Paylor, R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 5, 467–471 (2006).
Hayashi, M.L. et al. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc. Natl. Acad. Sci. USA 104, 11489–11494 (2007).
Zhao, M.G. et al. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J. Neurosci. 25, 7385–7392 (2005).
Krueger, D.D. & Bear, M.F. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu. Rev. Med. 62, 411–429 (2010).
Mercaldo, V., Descalzi, G. & Zhuo, M. Fragile X mental retardation protein in learning-related synaptic plasticity. Mol. Cells 28, 501–507 (2009).
Bassell, G.J. & Warren, S.T. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60, 201–214 (2008).
Luo, Y. et al. Fragile X mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 6, e1000898 (2010).
Mientjes, E.J. et al. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol. Dis. 21, 549–555 (2006).
Lagace, D.C. et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 27, 12623–12629 (2007).
Ables, J.L. et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J. Neurosci. 30, 10484–10492 (2010).
Raponi, E. et al. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia 55, 165–177 (2007).
Jin, P. & Warren, S.T. New insights into fragile X syndrome: from molecules to neurobehaviors. Trends Biochem. Sci. 28, 152–158 (2003).
Shors, T.J., Townsend, D.A., Zhao, M., Kozorovitskiy, Y. & Gould, E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584 (2002).
Peier, A.M. et al. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum. Mol. Genet. 9, 1145–1159 (2000).
Eadie, B.D. et al. Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol. Dis. 36, 361–373 (2009).
Spencer, C.M. et al. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X–related proteins. Hum. Mol. Genet. 15, 1984–1994 (2006).
Van Dam, D. et al. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav. Brain Res. 117, 127–136 (2000).
Misane, I. et al. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus 15, 418–426 (2005).
Moore, M.D. et al. Trace and contextual fear conditioning is enhanced in mice lacking the α4 subunit of the GABAA receptor. Neurobiol. Learn. Mem. 93, 383–387 (2010).
Quinn, J.J., Oommen, S.S., Morrison, G.E. & Fanselow, M.S. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace and delay fear conditioning in a temporally specific manner. Hippocampus 12, 495–504 (2002).
Gilbert, P.E., Kesner, R.P. & Lee, I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus 11, 626–636 (2001).
Aimone, J.B., Deng, W. & Gage, F.H. Adult neurogenesis: integrating theories and separating functions. Trends Cogn. Sci. 14, 325–337 (2010).
Aimone, J.B., Wiles, J. & Gage, F.H. Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 9, 723–727 (2006).
Feng, R. et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32, 911–926 (2001).
Stone, S.S. et al. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus published online, doi:10.1002/hipo.20845 (7 September 2010).
Toni, N. et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 11, 901–907 (2008).
Ge, S., Yang, C.H., Hsu, K.S., Ming, G.L. & Song, H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566 (2007).
Schmidt-Hieber, C., Jonas, P. & Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187 (2004).
Bhattacharyya, A. et al. Normal neurogenesis but abnormal gene expression in human fragile X cortical progenitor cells. Stem Cells Dev. 17, 107–117 (2008).
Castrén, M. et al. Altered differentiation of neural stem cells in fragile X syndrome. Proc. Natl. Acad. Sci. USA 102, 17834–17839 (2005).
Cunningham, C.L. et al. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum. Mol. Genet. 20, 64–79 (2010).
Pepper, A.S., Beerman, R.W., Bhogal, B. & Jongens, T.A. Argonaute2 suppresses Drosophila fragile X expression preventing neurogenesis and oogenesis defects. PLoS One 4, e7618 (2009).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Smrt, R.D. et al. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol. Dis. 27, 77–89 (2007).
Smrt, R.D. et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells 28, 1060–1070 (2010).
Babu, H., Cheung, G., Kettenmann, H., Palmer, T.D. & Kempermann, G. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS ONE 2, e388 (2007).
Barkho, B.Z. et al. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells 26, 3139–3149 (2008).
Liu, C. et al. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell 6, 433–444 (2010).
Kaspar, B.K. et al. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc. Natl. Acad. Sci. USA 99, 2320–2325 (2002).
Paz, R., Barsness, B., Martenson, T., Tanner, D. & Allan, A.M. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology 32, 693–699 (2007).
Martinez-Finley, E.J., Ali, A.M. & Allan, A.M. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels? Pharmacol. Biochem. Behav. 94, 271–277 (2009).
Harrell, A.V. & Allan, A.M. Improvements in hippocampal-dependent learning and decremental attention in 5-HT(3) receptor overexpressing mice. Learn. Mem. 10, 410–419 (2003).
Allan, A.M. et al. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Hum. Mol. Genet. 17, 2047–2057 (2008).
Acknowledgements
We thank C.T. Strauss for editing the manuscript; H. van Praag, D. Schaffer and T. Xie for critical reading of the manuscript; members of the Zhao lab for discussions; S.J. von Hoyningen-Huene for technical assistance; D.C. Lagace and R.D. Smrt for technical help; and H. van Praag (US National Institutes of Health (NIH)/National Institute of Aging) and F. Gage (Salk Institute) for the Cre-GFP construct. This work was supported by grants from the NIH to X.Z. (MH080434 and MH078972) and D.L.N. (HD38038 and HD024064). E.G.S. is supported by an NIH National Institute of Mental Health Career Opportunity for Research training grant (MH19101). Images in this paper were generated in the University of New Mexico Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed here: http://hsc.unm.edu/crtc/microscopy/acknowledgement.shtml/.
Author information
Authors and Affiliations
Contributions
W.G. and X.Z. planned the experiments, analyzed data and wrote the manuscript. A.M.A. developed, carried out and analyzed data for behavioral analyses. W.G., L.Z., E.B.J., E.G.S., A.C.M., S.L.G. and A.M.A. carried out all the experiments. P.J. helped with mouse line acquisition and concept development. R.Z., B.A.O. and D.L.N. made Fmr1-cON mice. A.J.E. provided the Nes-CreERT2 and ROSA26-YFP mouse lines and guided histological analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Methods (PDF 1372 kb)
Rights and permissions
About this article
Cite this article
Guo, W., Allan, A., Zong, R. et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat Med 17, 559–565 (2011). https://doi.org/10.1038/nm.2336
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2336