Abstract
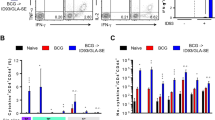
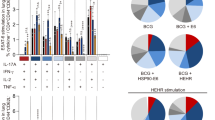
All tuberculosis vaccines currently in clinical trials are designed as prophylactic vaccines based on early expressed antigens. We have developed a multistage vaccination strategy in which the early antigens Ag85B and 6-kDa early secretory antigenic target (ESAT-6) are combined with the latency-associated protein Rv2660c (H56 vaccine). In CB6F1 mice we show that Rv2660c is stably expressed in late stages of infection despite an overall reduced transcription. The H56 vaccine promotes a T cell response against all protein components that is characterized by a high proportion of polyfunctional CD4+ T cells. In three different pre‐exposure mouse models, H56 confers protective immunity characterized by a more efficient containment of late-stage infection than the Ag85B-ESAT6 vaccine (H1) and BCG. In two mouse models of latent tuberculosis, we show that H56 vaccination after exposure is able to control reactivation and significantly lower the bacterial load compared to adjuvant control mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fine, P.E. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346, 1339–1345 (1995).
Andersen, P. Tuberculosis vaccines—an update. Nat. Rev. Microbiol. 5, 484–487 (2007).
Doherty, T.M., Dietrich, J. & Billeskov, R. Tuberculosis subunit vaccines: from basic science to clinical testing. Expert Opin. Biol. Ther. 7, 1539–1549 (2007).
Olsen, A.W., van Pinxteren, L.A.H., Okkels, L.M., Rasmussen, P.B. & Andersen, P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69, 2773–2778 (2001).
Skeiky, Y.A. et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172, 7618–7628 (2004).
Corbett, E.L. et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163, 1009–1021 (2003).
Wilkinson, D. & Davies, G.R. The increasing burden of tuberculosis in rural South Africa—impact of the HIV epidemic. S. Afr. Med. J. 87, 447–450 (1997).
Horwitz, M.A., Lee, B.W., Dillon, B.J. & Harth, G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92, 1530–1534 (1995).
Baldwin, S.L. et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66, 2951–2959 (1998).
Brandt, L., Elhay, M., Rosenkrands, I., Lindblad, E.B. & Andersen, P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68, 791–795 (2000).
Olsen, A.W., Williams, A., Okkels, L.M., Hatch, G. & Andersen, P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect. Immun. 72, 6148–6150 (2004).
Dietrich, J., Billeskov, R., Doherty, T.M. & Andersen, P. Synergistic effect of bacillus Calmette Guerin and a tuberculosis subunit vaccine in cationic liposomes: increased immunogenicity and protection. J. Immunol. 178, 3721–3730 (2007).
Langermans, J.A. et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 23, 2740–2750 (2005).
Agger, E.M. et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS ONE 3, e3116 (2008).
Dye, C. Global epidemiology of tuberculosis. Lancet 367, 938–940 (2006).
Finlay, B.B. & Falkow, S. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61, 136–169 (1997).
Voskuil, M.I., Visconti, K.C. & Schoolnik, G.K. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb.) 84, 218–227 (2004).
Rustad, T.R., Harrell, M.I., Liao, R. & Sherman, D.R. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS ONE 3, e1502 (2008).
Betts, J.C., Lukey, P.T., Robb, L.C., McAdam, R.A. & Duncan, K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43, 717–731 (2002).
Muttucumaru, D.G., Roberts, G., Hinds, J., Stabler, R.A. & Parish, T. Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinb.) 84, 239–246 (2004).
Schnappinger, D. et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198, 693–704 (2003).
Butcher, P.D., Mangan, J.A. & Monahan, I.M. Intracellular gene expression. Analysis of RNA from mycobacteria in macrophages using RT-PCR. Methods Mol. Biol. 101, 285–306 (1998).
Dolganov, G.M. et al. A novel method of gene transcript profiling in airway biopsy homogenates reveals increased expression of a Na+-K+-Cl− cotransporter (NKCC1) in asthmatic subjects. Genome Res. 11, 1473–1483 (2001).
Brosch, R. et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. USA 104, 5596–5601 (2007).
Lin, M.Y. et al. Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccination. Infect. Immun. 75, 3523–3530 (2007).
McCune, R.M. Jr., McDermott, W. & Tompsett, R. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J. Exp. Med. 104, 763–802 (1956).
Manganelli, R., Voskuil, M.I., Schoolnik, G.K. & Smith, I. The Mycobacterium tuberculosis ECF σ factor σE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41, 423–437 (2001).
Fontán, P.A. et al. Mycobacterium tuberculosis σ factor E regulon modulates the host inflammatory response. J. Infect. Dis. 198, 877–885 (2008).
Voskuil, M.I. et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198, 705–713 (2003).
Govender, L. et al. Higher human CD4 T cell response to novel Mycobacterium tuberculosis latency associated antigens Rv2660 and Rv2659 in latent infection compared with tuberculosis disease. Vaccine 29, 51–57 (2010).
Dietrich, J. et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J. Immunol. 174, 6332–6339 (2005).
Palendira, U., Spratt, J.M., Britton, W.J. & Triccas, J.A. Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol Mycobacterium tuberculosis infection. Vaccine 23, 1680–1685 (2005).
Darrah, P.A. et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13, 843–850 (2007).
Wille-Reece, U. et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 203, 1249–1258 (2006).
Beveridge, N.E. et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis–specific CD4+ memory T lymphocyte populations. Eur. J. Immunol. 37, 3089–3100 (2007).
Kannanganat, S., Ibegbu, C., Chennareddi, L., Robinson, H.L. & Amara, R.R. Multiple-cytokine–producing antiviral CD4 T cells are functionally superior to single-cytokine–producing cells. J. Virol. 81, 8468–8476 (2007).
Kannanganat, S. et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J. Virol. 81, 12071–12076 (2007).
Heeney, J.L. & Plotkin, S.A. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 7, 1281–1284 (2006).
Lindenstrøm, T. et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182, 8047–8055 (2009).
Acknowledgements
We thank the Bill and Melinda Gates Foundation (GC12 #37885), EC-FP6: TB-VAC (LSHP-CT-2003-503367), the US National Institutes of Health and National Institute of Allergy and Infectious Diseases (HHSN266200400091c) and the TB Vaccine Testing and Research Materials Contract (NOI-AI-40091). Thanks to N. Caceres for her contribution on the model of latent tuberculosis, to L. Rasmussen, M. Henriksen, J. Brady and V. Andersen for excellent technical help and to E.M. Agger for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
C.A. conceived of the study, produced H56, conducted pre‐exposure vaccine studies and prepared the manuscript. T.H. developed the CB6F1 latency model and conducted latency studies in this model. J.D. conducted the BCG boost studies. P.-J.C. developed the C57BL/6 latency model and conducted latency studies in this model. A.I. conducted pre‐exposure vaccine studies. G.D. designed and performed gene expression analyses. G.K.S. designed and performed gene expression analyses. J.P.C. performed histological evaluation of lung specimens. R.B. contributed to the latency vaccine studies. P.A. conceived of the study and prepared the manuscript. All authors discussed the results and commented on the manuscript at all stages.
Corresponding authors
Ethics declarations
Competing interests
C.A. and P.A. are co-inventors on a patent application to the Danish patent office covering the use of H56 as a vaccine. All rights have been assigned to Statens Serum Institut, a Danish not-for-profit governmental institute.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 307 kb)
Rights and permissions
About this article
Cite this article
Aagaard, C., Hoang, T., Dietrich, J. et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 17, 189–194 (2011). https://doi.org/10.1038/nm.2285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2285
This article is cited by
-
Production and Evaluation of Ag85B:HspX:hFcγ1 Immunogenicity as an Fc Fusion Recombinant Multi-Stage Vaccine Candidate Against Mycobacterium tuberculosis
Current Microbiology (2024)
-
Intranasal multivalent adenoviral-vectored vaccine protects against replicating and dormant M.tb in conventional and humanized mice
npj Vaccines (2023)
-
Peptide microarray-based identification of dormancy-associated Mycobacterium tuberculosis antigens inducing immune responses among latent tuberculosis infection individuals in Thailand
Scientific Reports (2023)
-
Cerium Nanoparticles Can Enhance Humoral and Cellular Immune Responses of Multi-epitope Vaccine Candidates
BioNanoScience (2023)
-
Transmission Dynamics of Tuberculosis with Age-specific Disease Progression
Bulletin of Mathematical Biology (2022)