Abstract
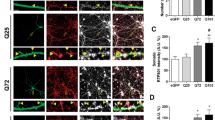
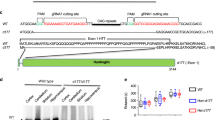
Huntington's disease is caused by an expanded CAG repeat in the gene encoding huntingtin (HTT), resulting in loss of striatal and cortical neurons. Given that the gene product is widely expressed, it remains unclear why neurons are selectively targeted. Here we show the relationship between synaptic and extrasynaptic activity, inclusion formation of mutant huntingtin protein (mtHtt) and neuronal survival. Synaptic N-methyl-D-aspartate–type glutamate receptor (NMDAR) activity induces mtHtt inclusions via a T complex-1 (TCP-1) ring complex (TRiC)-dependent mechanism, rendering neurons more resistant to mtHtt-mediated cell death. In contrast, stimulation of extrasynaptic NMDARs increases the vulnerability of mtHtt-containing neurons to cell death by impairing the neuroprotective cyclic AMP response element–binding protein (CREB)–peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α) cascade and increasing the level of the small guanine nucleotide–binding protein Rhes, which is known to sumoylate and disaggregate mtHtt. Treatment of transgenic mice expressing a yeast artificial chromosome containing 128 CAG repeats (YAC128) with low-dose memantine blocks extrasynaptic (but not synaptic) NMDARs and ameliorates neuropathological and behavioral manifestations. By contrast, high-dose memantine, which blocks both extrasynaptic and synaptic NMDAR activity, decreases neuronal inclusions and worsens these outcomes. Our findings offer a rational therapeutic approach for protecting susceptible neurons in Huntington's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Ciechanover, A. & Brundin, P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40, 427–446 (2003).
Beal, M.F. et al. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 321, 168–171 (1986).
Ferrante, R.J. et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J. Neurosci. 22, 1592–1599 (2002).
Zeron, M.M. et al. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33, 849–860 (2002).
Heng, M.Y., Detloff, P.J., Wang, P.L., Tsien, J.Z. & Albin, R.L. In vivo evidence for NMDA receptor–mediated excitotoxicity in a murine genetic model of Huntington disease. J. Neurosci. 29, 3200–3205 (2009).
Slow, E.J. et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum. Mol. Genet. 12, 1555–1567 (2003).
Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 5, 160–170 (2006).
Lipton, S.A. Pathologically activated therapeutics for neuroprotection. Nat. Rev. Neurosci. 8, 803–808 (2007).
Hardingham, G.E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Li, L., Murphy, T.H., Hayden, M.R. & Raymond, L.A. Enhanced striatal NR2B-containing N-methyl-D-aspartate receptor–mediated synaptic currents in a mouse model of Huntington disease. J. Neurophysiol. 92, 2738–2746 (2004).
Pickett, J. Folding away the bad guys. Nat. Rev. Neurosci. 7, 832–833 (2006).
Tam, S., Geller, R., Spiess, C. & Frydman, J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat. Cell Biol. 8, 1155–1162 (2006).
Melville, M.W., McClellan, A.J., Meyer, A.S., Darveau, A. & Frydman, J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol. Cell. Biol. 23, 3141–3151 (2003).
Behrends, C. et al. TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol. Cell 23, 887–897 (2006).
Kitamura, A. et al. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat. Cell Biol. 8, 1163–1170 (2006).
King, M.A. et al. Cytoplasmic inclusions of Htt exon1 containing an expanded polyglutamine tract suppress execution of apoptosis in sympathetic neurons. J. Neurosci. 28, 14401–14415 (2008).
Davies, S.W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997).
Saudou, F., Finkbeiner, S., Devys, D. & Greenberg, M.E. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55–66 (1998).
Tsai, J., Grutzendler, J., Duff, K. & Gan, W.B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 7, 1181–1183 (2004).
Arrasate, M., Mitra, S., Schweitzer, E.S., Segal, M.R. & Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810 (2004).
Sánchez, I., Mahlke, C. & Yuan, J. Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 421, 373–379 (2003).
Friedlander, R.M. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 348, 1365–1375 (2003).
Tovar, K.R. & Westbrook, G.L. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 19, 4180–4188 (1999).
Lipton, S.A. & Rosenberg, P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 330, 613–622 (1994).
Fellin, T. et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743 (2004).
Tian, G.F. et al. An astrocytic basis of epilepsy. Nat. Med. 11, 973–981 (2005).
Subramaniam, S., Sixt, K.M., Barrow, R. & Snyder, S.H. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science 324, 1327–1330 (2009).
Nucifora, F.C., Jr. et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291, 2423–2428 (2001).
McGill, J.K. & Beal, M.F. PGC-1α, a new therapeutic target in Huntington's disease? Cell 127, 465–468 (2006).
Chen, H.S. et al. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 12, 4427–4436 (1992).
Hesselink, M.B., De Boer, B.G., Breimer, D.D. & Danysz, W. Brain penetration and in vivo recovery of NMDA receptor antagonists amantadine and memantine: a quantitative microdialysis study. Pharm. Res. 16, 637–642 (1999).
Parsons, C.G., Stöffler, A. & Danysz, W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology 53, 699–723 (2007).
Gutekunst, C.A. et al. Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J. Neurosci. 19, 2522–2534 (1999).
Vonsattel, J.P. et al. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 44, 559–577 (1985).
Cui, L. et al. Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127, 59–69 (2006).
Papadia, S., Stevenson, P., Hardingham, N.R., Bading, H. & Hardingham, G.E. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J. Neurosci. 25, 4279–4287 (2005).
Soriano, F.X. et al. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J. Neurosci. 26, 4509–4518 (2006).
Beister, A. et al. The N-methyl-D-aspartate antagonist memantine retards progression of Huntington's disease. J. Neural Transm. Suppl. 68, 117–122 (2004).
Okamoto, S.-i. et al. Dominant-interfering forms of MEF2 generated by caspase cleavage contribute to NMDA-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 99, 3974–3979 (2002).
Lei, S.Z. et al. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron 8, 1087–1099 (1992).
Van Raamsdonk, J.M. et al. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J. Neurosci. 25, 4169–4180 (2005).
Acknowledgements
We thank C.A. Ross (Johns Hopkins University School of Medicine) and L.M. Ellerby (Buck Institute for Age Research) for N-terminal and full-length constructs of huntingtin, T.F. Newmeyer and H. Fang (Burnham Institute for Medical Research) for preparing primary neuronal cultures, X.-J. Li (Emory University School of Medicine) for providing the EM48 antibody and E. Bossy-Wetzel, Y.S. Choo, W. Zago and T. Nakamura for assistance or discussion. This work was supported in part by US National Institutes of Health grants P01 HD29587, P01 ES016738, R01 EY09024, R01 EY05477 and R01 NS41207 and a Senior Scholar Award in Aging Research from the Ellison Medical Foundation (S.A.L.). Additional support was provided by the National Institutes of Health Blueprint Grant for La Jolla Interdisciplinary Neuroscience Center Cores P30 NS057096. M.A.P. was supported by the Canadian Institute of Health Research and the Michael Smith Foundation for Health Research. M.R.H. was supported by grants from the Canadian Institutes of Health Research, the Huntington Society of Canada, the Huntington's Disease Society of America, CHDI Foundation, Inc., and the HighQ Foundation.
Author information
Authors and Affiliations
Contributions
S.-i.O. and D.Y. designed and performed the in vitro experiments. R.Z. and A.C. assisted with the in vitro experiments. M.K. offered key advice and helped analyze the in vitro experiments on mtHtt inclusions and cell death. M.A.P., D.E.E. and R.K.G. designed and conducted the mouse studies. M.R.H. conceptualized and supervised the mouse studies. M.T. and P.X. performed the electrophysiology experiments. D.Z., H.-S.V.C., G.T. and S.A.L. supervised the electrophysiological experiments and gave crucial advice. S.-i.O., M.A.P., M.T., D.Y. and M.R.H. wrote the first draft of the manuscript. S.-i.O. and S.A.L. formulated the hypothesis, conceptualized the entire study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.L. is the named inventor on numerous patents in territories worldwide for the use of memantine in neurodegenerative disorders. He has no direct ownership in the drug, which is currently clinically approved and marketed for moderate to severe Alzheimer’s disease under the name Namenda. Under the rules of Harvard University, his former institution where the work was initially performed, S.L. participates in a royalty-sharing plan administered by Harvard Medical School and Children’s Hospital, Boston.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18 and Supplementary Methods (PDF 1518 kb)
Rights and permissions
About this article
Cite this article
Okamoto, Si., Pouladi, M., Talantova, M. et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med 15, 1407–1413 (2009). https://doi.org/10.1038/nm.2056
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2056
This article is cited by
-
Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Targeted protein S-nitrosylation of ACE2 inhibits SARS-CoV-2 infection
Nature Chemical Biology (2023)
-
Krankheitsmodifizierende Therapieansätze bei der Huntington-Krankheit
Der Nervenarzt (2022)
-
Mutant Huntingtin stalls ribosomes and represses protein synthesis in a cellular model of Huntington disease
Nature Communications (2021)
-
NitroSynapsin ameliorates hypersynchronous neural network activity in Alzheimer hiPSC models
Molecular Psychiatry (2021)