Abstract
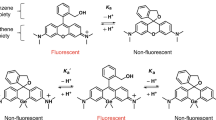
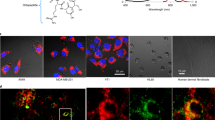
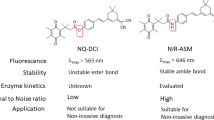
A long-term goal of cancer diagnosis is to develop tumor-imaging techniques that have sufficient specificity and sensitivity. To achieve this goal, minimizing the background signal originating from nontarget tissues is crucial. Here we achieve highly specific in vivo cancer visualization by using a newly designed targeted 'activatable' fluorescent imaging probe. This agent is activated after cellular internalization by sensing the pH change in the lysosome. Novel acidic pH–activatable probes based on the boron-dipyrromethene fluorophore were synthesized and then conjugated to a cancer-targeting monoclonal antibody. As proof of concept, ex vivo and in vivo imaging of human epidermal growth factor receptor type 2–positive lung cancer cells in mice was performed. The probe was highly specific for tumors with minimal background signal. Furthermore, because the acidic pH in lysosomes is maintained by the energy-consuming proton pump, only viable cancer cells were successfully visualized. The design concept can be widely adapted to cancer-specific, cell surface–targeting molecules that result in cellular internalization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yamamoto, N. et al. Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Res. 64, 4251–4256 (2004).
Yamauchi, K. et al. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 68, 516–520 (2008).
Yang, M., Jiang, P. & Hoffman, R.M. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 67, 5195–5200 (2007).
Wu, A.M. & Senter, P.D. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 23, 1137–1146 (2005).
Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 37, Suppl 4, S3–S8 (2001).
Miura, T. et al. Rational design principle for modulating fluorescence properties of fluorescein-based probes by photoinduced electron transfer. J. Am. Chem. Soc. 125, 8666–8671 (2003).
Ueno, T. et al. Rational principles for modulating fluorescence properties of fluorescein. J. Am. Chem. Soc. 126, 14079–14085 (2004).
Tanaka, K. et al. Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J. Am. Chem. Soc. 123, 2530–2536 (2001).
Urano, Y. et al. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J. Am. Chem. Soc. 127, 4888–4894 (2005).
Gabe, Y., Urano, Y., Kikuchi, K., Kojima, H. & Nagano, T. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophore - rational design of potentially useful bioimaging fluorescence probe. J. Am. Chem. Soc. 126, 3357–3367 (2004).
Queen, C. et al. A humanized antibody that binds to the interleukin 2 receptor. Proc. Natl. Acad. Sci. USA 86, 10029–10033 (1989).
Minta, A., Kao, J.P.Y. & Tsien, R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 264, 8171–8178 (1989).
Weissleder, R., Tung, C.H., Mahmood, U. & Bogdanov, A. Jr. In vivo imaging of tumors with proteaseactivated near-infrared fluorescent probes. Nat. Biotechnol. 17, 375–378 (1999).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284 (2000).
Paradiso, A.M., Tsien, R.Y. & Machen, T.E. Na+-H+ exchange in gastric glands as measured with a cytoplasmic-trapped, fluorescent ph indicator. Proc. Natl. Acad. Sci. USA 81, 7436–7440 (1984).
de Silva, A.P. et al. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997).
Kollmannsberger, M., Rurack, K., Resch-Genger, U. & Daub, J. Ultrafast charge transfer in amino-substituted boron dipyrromethene dyes and its inhibition by cation complexation: a new design concept for highly sensitive fluorescent probes. J. Phys. Chem. A 102, 10211–10220 (1998).
Adie, E.J. et al. A pH-sensitive fluor, CypHer™ 5, used to monitor agonist-induced G protein-coupled receptor internalization in live cells. Biotechniques 33, 1152–1154 (2002).
Nwokolo, C.U., Payne-James, J.J., Silk, D.B., Misiewicz, J.J. & Loft, D.E. Palliation of malignant dysphagia by ethanol induced tumour necrosis. Gut 35, 299–303 (1994).
Hama, Y. et al. A target-cell specific activatable fluorescence probe for in vivo molecular imaging of cancer based on a self-quenched avidin-rhodamine conjugate. Cancer Res. 67, 2791–2799 (2007).
Kamiya, M. et al. An enzymatically activated fluorescence probe for targeted tumor imaging. J. Am. Chem. Soc. 129, 3918–3929 (2007).
Hoffman, R.M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer 5, 796–806 (2005).
Baselga, J., Norton, L., Albanell, J., Kim, Y.M. & Mendelsohn, J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 58, 2825–2831 (1998).
Gancberg, D. et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann. Oncol. 13, 1036–1043 (2002).
Zidan, J. et al. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br. J. Cancer 93, 552–556 (2005).
Mandler, R., Kobayashi, H., Hinson, E.R., Brechbiel, M.W. & Waldmann, T.A. Herceptin-geldanamycin immunoconjugates: pharmacokinetics, biodistribution, and enhanced antitumor activity. Cancer Res. 64, 1460–1467 (2004).
Hama, Y., Urano, Y., Koyama, Y., Choyke, P.L. & Kobayashi, H. D-galactose receptor–targeted in vivo spectral fluorescence imaging of peritoneal metastasis using galactosamin-conjugated serum albumin-rhodamine green. J. Biomed. Opt. 12, 051501 (2007).
Alencar, H. et al. Colonic adenocarcinomas: near-infrared microcatheter imaging of smart probes for early detection—study in mice. Radiology 244, 232–238 (2007).
De Grand, A.M. & Frangioni, J.V. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol. Cancer Res. Treat. 2, 553–562 (2003).
Parker, C.A. & Rees, W.T. Correction of fluorescence spectra and measurement of fluorescence quantum efficiency. Analyst 85, 587–600 (1960).
Koyama, Y., Hama, Y., Urano, Y., Nguyen, D.M., Choyke, P.L. & Kobayashi, H. Spectral fluorescence molecular imaging of lung metastases targeting HER2/neu. Clin. Cancer Res. 13, 2936–2945 (2007).
Levenson, R.M. & Mansfield, J.R. Multispectral imaging in biology and medicine: slices of life. Cytometry A. 69, 748–758 (2006).
Acknowledgements
This study was supported in part by the Precursory Research for Embryonic Sciences and Technology from the Japan Science and Technology Agency, by research grants 19021010 and 19205021 to Y.U. and by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research to Y.H., Y.K., T.B., P.L.C. and H.K.
Author information
Authors and Affiliations
Contributions
Y.U. and H.K. planned the projects, developed the probes, performed the in vivo experiments and wrote and edited the manuscript. D.A. and M.K. developed the probes, performed the in vivo experiments and wrote and edited the manuscript. Y.H., Y.K., T.B., T.W. and A.H. performed the in vivo experiments and wrote and edited the manuscript. T.N. and P.L.C. planned the projects and wrote and edited the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4, Supplementary Tables 1–3 and Supplementary Methods (PDF 563 kb)
Supplementary Video 1
Reversible activation by altering the pH. (AVI 4293 kb)
Supplementary Video 2
Fluorescence-guided Laparoscope (MOV 12792 kb)
Supplementary Video 3
Fluorescence-guided Laparoscope (MOV 5792 kb)
Supplementary Video 4
Fluorescence-guided Laparoscope (MOV 8635 kb)
Supplementary Video 5
pH-activatable rhodamine (MOV 2877 kb)
Rights and permissions
About this article
Cite this article
Urano, Y., Asanuma, D., Hama, Y. et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med 15, 104–109 (2009). https://doi.org/10.1038/nm.1854
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1854
This article is cited by
-
Ratiometric fluorescent probe based on non-conjugated polymer dots for pH measurements in ordinary Portland cement-based materials
Microchimica Acta (2023)
-
The Evolution of Fluorescence-Guided Surgery
Molecular Imaging and Biology (2023)
-
Fundamentals and developments in fluorescence-guided cancer surgery
Nature Reviews Clinical Oncology (2022)
-
A phosphorescent probe for in vivo imaging in the second near-infrared window
Nature Biomedical Engineering (2021)
-
Molecular imaging and disease theranostics with renal-clearable optical agents
Nature Reviews Materials (2021)