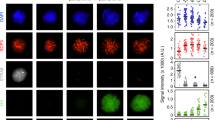
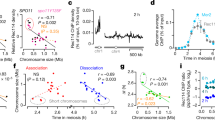
Abstract
Meiosis reduces the number of chromosomes carried by a diploid organism by half, partitioning precisely one haploid genome into each gamete. The basic events of meiosis reflect three meiosis-specific processes: first, pairing and synapsis of homologous chromosomes; second, high-frequency, precisely controlled, reciprocal crossover; third, the regulation of sister-chromatid cohesion (SCC), such that during anaphase I, SCC is released along the chromosome arms, but not at the centromeres. The failure of any of these processes can result in aneuploidy or a failure of meiotic segregation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nicklas, R.B. Chromosome segregation mechanisms. Genetics 78, 205–213 (1974).
Page, S.L. & Hawley, R.S. c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev. 15, 3130–3143 (2001).
Rockmill, B. & Roeder, G.S. Meiosis in asynaptic yeast. Genetics 126, 563–574 (1990).
Baudat, F. et al. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell 6, 989–998 (2000).
Grelon, M. et al. AtSPO11-1 is necessary for efficient meiotic recombination in plants. EMBO J. 20, 589–600 (2001).
Hawley, R.S. & Arbel, T. Yeast genetics and the fall of the classical view of meiosis. Cell 72, 301–303 (1993).
McKim, K.S. et al. Meiotic synapsis in the absence of recombination. Science 279, 876–878 (1998).
McKim, K.S. & Hayashi-Hagihara, A. mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev. 12, 2932–2942 (1998).
Dernburg, A.F. et al. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94, 387–398 (1998).
Walker, M.Y. & Hawley, R.S. Hanging on to your homolog: the roles of pairing, synapsis and recombination in the maintenance of homolog adhesion. Chromosoma 109, 3–9 (2000).
Cervantes, M.D., Farah, J.A. & Smith G.R. Meiotic DNA breaks associated with recombination in S. pombe. Mol. Cell 5 883–888 (2000).
Rasmusson, K. The transformation of the Synaptonemal Complex into the 'elimination chromatin' in Bombyx mori oocytes. Chromosoma 60, 205–221 (1977).
Zickler, D. & Kleckner, N. Meiotic chromosomes: integrating structure and function. Annu. Rev.Genet. 33, 603–754 (1999).
Keeney, S., Giroux, C.N. & Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88, 375–384 (1997).
Romanienko, P.J. & Camerini-Otero, R.D. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 6, 975–987 (2000).
Szostak, J.W. et al. The double-strand-break repair model for recombination. Cell 33, 25–35 (1983).
Schwacha, A. & Kleckner, N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell 76, 51–63 (1994).
Schwacha, A. & Kleckner, N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83, 783–791 (1995).
Allers, T. & Lichten, M. Intermediates of yeast meiotic recombination contain heteroduplex DNA. Mol. Cell 8 225–231 (2001).
Cromie, G.A. & Leach, D.R. Control of crossing over. Mol. Cell 6, 815–826 (2000).
Zwick, M.E., Cutler, D.J. & Langley, C.H. Classic Weinstein: tetrad analysis, genetic variation and achiasmate segregation in Drosophila and humans. Genetics 152, 1615–1629 (1999).
Jones, G.H. The control of chiasma distribution. Symp. Soc. Exp. Biol. 38, 293–320 (1984).
Carpenter, A.T.C. Genetic Recombination (ed. Kucherlapati, R.) 529–549 (ASM Press, Washington DC, 1988).
Hulten, M. Chiasma formation, crossing-over and recombination in meiosis. Trends Genet. 10, 112–115 (1994).
Lichten, M. & Haber, J.E. Position effects in ectopic and allelic mitotic recombination in Saccharomyces cerevisiae. Genetics 123, 261–268 (1989).
Burgoyne, P.S. Mammalian X and Y crossover. Nature 319, 258–259 (1986).
Novak, J.E., Ross-Macdonald, P.B. & Roeder, G.S. The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 158, 1013–1025 (2001).
Page, S.L. et al. Genetic studies of mei-P26 reveal a link between the processes that control germ cell proliferation in both sexes and those that control meiotic exchange in Drosophila. Genetics 155, 1757–1772 (2000).
McKim, K.S. & Hawley, R.S. Chromosomal control of meiotic cell division. Science 270, 1595–1601 (1995).
Nicklas, R.B. Chromosome distribution: experiments on cell hybrids and in vitro. Philos. Trans. R. Soc. Lond. B 277, 267–276 (1977).
Nicklas, R.B. & Staehly, C.A. Chromosome micromanipulation. I. The mechanics of chromosome attachment to the spindle. Chromosoma 21, 1–16 (1967).
Hassold, T. & Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Rev. Genet. 2, 280–291 (2001).
Theurkauf, W.E. & Hawley, R.S. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116, 1167–1180 (1992).
Darlington, C.D. Recent Advances in Cytology (The Blakiston Company, Philadelphia, 1932).
Hawley, R.S. Genetic Recombination (ed. Kucherlapati, R.) 497–528 (ASM Press, Washington DC, 1988).
Lee, J.Y. & Orr-Weaver, T.L. The molecular basis of sister-chromatid cohesion. Annu. Rev. Cell Dev. Biol. 17, 753–777 (2001).
Maguire, M.P., Paredes, A.M. & Riess, R.W. The desynaptic mutant of maize as a combined defect of synaptonemal complex and chiasma maintenance. Genome 34, 879–887 (1991).
Maguire, M. Evidence for separate control of crossing over and chiasma maintenance in maize. Chromosoma 65, 173–183 (1978).
Maguire, M.P. The need for a chiasma binder. J. Theor. Biol. 48, 485–487 (1974).
Maguire, M.P. A possible role for the synaptonemal complex in chiasma maintenance. Exp. Cell Res. 112, 297–308 (1978).
Bickel, S.E. et al. Genetic interactions between mei-S332 and ord in the control of sister-chromatid cohesion. Genetics 150, 1467–1476 (1998).
Buonomo, S.B. et al. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103, 387–398 (2000).
Hartman, T. et al. Pds5p is an essential chromosomal protein required for both sister chromatid cohesion and condensation in Saccharomyces cerevisiae. J. Cell Biol. 151, 613–626 (2000).
van Heemst, D. & Heyting, C. Sister chromatid cohesion and recombination in meiosis. Chromosoma 109, 10–26 (2000).
Warren, W.D. et al. The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr. Biol. 10, 1463–1466 (2000).
Shonn, M.A., McCarroll, R. & Murray, A.W. Spo13 protects meiotic cohesin at centromeres in meiosis I. Genes Dev. 16, 1659–1671 (2002).
Toth, A. et al. Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis I. Cell 103, 1155–1168 (2000).
Watanabe, Y. & Nurse, P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400, 461–464 (1999).
Orr-Weaver, T.L. Meiosis in Drosophila: seeing is believing. Proc. Natl Acad. Sci. USA 92, 10443–10449 (1995).
Shonn, M.A., McCarroll, R. & Murray, A.W. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science 289, 300–303 (2000).
Nicklas, R.B., Ward, S.C. & Gorbsky, G.J. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol. 130, 929–939 (1995).
LeMaire-Adkins, R., Radke, K. & Hunt, P.A. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J. Cell Biol. 139, 1611–1619 (1997).
Pelttari, J. et al. A meiotic chromosomal core consisting of cohesin complex proteins recruits DNA recombination proteins and promotes synapsis in the absence of an axial element in mammalian meiotic cells. Mol. Cell Biol. 21, 5667–5677 (2001).
Hassold, T.J. et al. XY chromosome nondisjunction in man is associated with diminished recombination in the pseudoautosomal region. Am. J. Hum. Genet. 49, 253–260 (1991).
Koehler, K.E. et al. Spontaneous X chromosome MI and MII nondisjunction events in Drosophila melanogaster oocytes have different recombinational histories. Nature Genet. 14, 406–414 (1996).
Lamb, N.E. et al. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nature Genet. 14, 400–405 (1996).
Hassold, T., S. Sherman, & P.A. Hunt, The origin of trisomy in humans. Prog. Clin. Biol. Res. 393, 1–12 (1995).
MacDonald, M. et al. The origin of 47,XXY and 47,XXX aneuploidy: heterogeneous mechanisms and role of aberrant recombination. Hum. Mol. Genet. 3, 1365–1371 (1994).
Lorda-Sanchez, I. et al. Molecular study of 45,X conceptuses: correlation with clinical findings. Am. J. Med. Genet. 42, 487–490 (1992).
Hassold, T. et al. Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am. J. Hum. Genet. 57, 867–874 (1995).
Ross, L.O., Maxfield, R. & Dawson, D. Exchanges are not equally able to enhance meiotic chromosome segregation in yeast. Proc. Natl Acad. Sci. USA 93, 4979–4983 (1996).
Carpenter, A.T. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73, 393–428 (1973).
Zitron, A.E. & Hawley, R.S. The genetic analysis of distributive segregation in Drosophila melanogaster. I. Isolation and characterization of Aberrant X segregation (Axs), a mutation defective in chromosome partner choice. Genetics 122, 801–821 (1989).
Rasooly, R.S. et al. The lethal(1)TW-6cs mutation of Drosophila melanogaster is a dominant antimorphic allele of nod and is associated with a single base change in the putative ATP-binding domain. Genetics 129, 409–422 (1991).
Hawley, R.S. & Theurkauf, W.E. Requiem for the distributive system: Achiasmate segregation in Drosophila females. Trends Genet. 9, 310–317 (1993).
Wolf, K.W. How meiotic cells deal with non-exchange chromosomes. Bioessays 16, 107–114 (1994).
McKim, K.S. & Rose, A.M. Chromosome I duplications in Caenorhabditis elegans. Genetics 124, 115–132 (1990).
Dawson, D.S., Murray, A.W. & Szostak, J.W. An alternative pathway for meiotic chromosome segregation in yeast. Science 234, 713–717 (1986).
Molnar, M. et al. Live observation of fission yeast meiosis in recombination-deficient mutants: a study on achiasmate chromosome segregation. J. Cell Sci. 114, 2843–2853 (2001).
Green-Marroquin, B.L. et al. Orientation of nonrandomly segregating sex chromosomes in spermatocytes of the flea beetle, Alagoasa bicolor L. Chromosoma. 110, 32–38 (2001).
Dernburg, A.F., Sedat, J.W. & Hawley, R.S. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86, 135–146 (1996).
Hawley, R.S. et al. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13, 440–467 (1992).
Matthies, H.J., Baskin, R.J. & Hawley, R.S. Orphan Kinesin NOD Lacks Motile Properties But Does Possess a Microtubule-stimulated ATPase Activity. Mol. Biol. Cell 12, 4000–4012 (2001).
Loidl, J., Scherthan, H. & Kaback, D.B. Physical association between nonhomologous chromosomes precedes distributive disjunction in yeast. Proc. Natl Acad. Sci. USA 91, 331–334 (1994).
Lynn, A. et al. Patterns of meiotic recombination on the long arm of human chromosome 21. Genome Res. 10, 1319–1332 (2000).
Tease, C., Hartshorne, G.M. & Hulten, M.A. Patterns of meiotic recombination in human fetal oocytes. Am. J. Hum. Genet. 70, 1469–1479 (2002).
Sears, E.R. Misdivision of univalents in common wheat. Chromosoma 4, 535–550 (1952).
Orr-Weaver, T. Meiotic nondisjunction does the two-step. Nature Genet. 14, 374–376 (1996).
Hawley, R.S., Frazier, J.A. & Rasooly, R. Separation anxiety: the etiology of nondisjunction in flies and people. Hum. Mol. Genet. 3, 1521–1528 (1994).
Koehler, K.E. et al. Recombination and nondisjunction in humans and flies. Hum. Mol. Genet. 5, 1495–1504 (1996).
Eichenlaub-Ritter, U. Genetics of oocyte ageing. Maturitas 30, 143–169 (1998).
Freeman, S.B. et al. Women with a reduced ovarian complement may have an increased risk for a child with Down syndrome. Am. J. Hum. Genet. 66, 1680–1683 (2000).
Sekelsky, J.J. et al. Identification of novel Drosophila meiotic genes recovered in a P- element screen. Genetics 152 529–542 (1999).
Kline, J. et al. Trisomic pregnancy and earlier age at menopause. Am. J. Hum. Genet. 67 395–404 (2000).
Angell, R. First-meiotic-division nondisjunction in human oocytes. Am. J. Hum. Genet. 61, 23–32 (1997).
Acknowledgements
We thank T. Hassold and members of the Hawley laboratory for critical comments on the text. We also thank K. McKim and K. Koehler for providing us with the initials drafts of Figs 3 and 4, respectively. Finally, we are grateful to one anonymous reviewer, whose constructive commentary aided us enormously.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Champion, M., Hawley, R. Playing for half the deck: the molecular biology of meiosis. Nat Med 8 (Suppl 10), S50–S56 (2002). https://doi.org/10.1038/nm-fertilityS50
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm-fertilityS50