Abstract
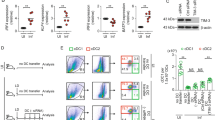
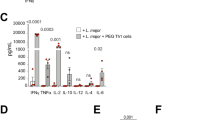
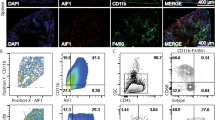
Interaction between dendritic cells (DCs) and T cells is essential for the generation of cell-mediated immunity. Here we show that DCs from mice with chronic Leishmania donovani infection fail to migrate from the marginal zone to the periarteriolar region of the spleen. Stromal cells were fewer, which was associated with loss of CCL21 and CCL19 expression. The residual stromal cells and endothelium produced sufficient CCL21 to direct the migration of DCs transferred from naïve mice. However, DCs from infected mice had impaired migration both in naïve recipients and in vitro, in response to CCL21 and CCL19. Defective localization was attributable to tumor necrosis factor-α–dependent, interleukin 10–mediated inhibition of CCR7 expression. Effective immunotherapy was achieved with CCR7-expressing DCs, without the need to identify protective Leishmania antigens. Thus defective DC migration plays a major role in the pathogenesis of this disease and the immunosuppression is mediated, at least in part, through the spatial segregation of DCs and T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Jenkins, M.K. et al. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19, 23–45 (2001).
Zlotnik, A. & Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 (2000).
Cyster, J.G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
Kellermann, S.A., Hudak, S., Oldham, E.R., Liu, Y.J. & McEvoy, L.M. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3β are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J. Immunol. 162, 3859–3864 (1999).
Luther, S.A., Tang, H.L., Hyman, P.L., Farr, A.G. & Cyster, J.G. Co-expression of the chemokines ELC and SLC by T cell zone stromal cells and deletion of the ELC gene in the plt/plt mice. Proc. Natl. Acad. Sci. USA 97, 12694–12699 (2000).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Gunn, M.D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999).
Ngo, V.N. et al. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 (1999).
Ngo, V.N., Cornall, R.J. & Cyster, J.G. Splenic T zone development is B cell dependent. J. Exp. Med. 194, 1649–1660 (2001).
Engwerda, C.R. et al. Remodelling of the splenic marginal zone macrophages following during Leishmania donovani infection by TNF-α. Am. J. Pathol. 161, 429–437 (2002).
Odermatt, B., Eppler, M., Leist, T.P., Hengartner, H. & Zinkernagel, R.M. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl. Acad. Sci. USA. 88, 8252–8256 (1991).
Ho, M., Koech, D.K., Iha, D.W. & Bryceson, A.D. Immunosuppression in Kenyan visceral leishmaniasis. Clin. Exp. Immunol. 51, 207–214 (1983).
Engwerda, C.R. & Kaye, P.M. Organ-specific immune responses associated with infectious diseases. Immunol. Today 21, 73–77 (2000).
Murray, H.W. et al. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J. Immunol. 148, 1858–1863 (1992).
Engwerda, C.R., Murphy, M.L., Cotterell, S.E., Smelt, S.C. & Kaye, P.M. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur. J. Immunol. 28, 669–680 (1998).
Smelt, S.C., Engwerda, C.R., McCrossen, M. & Kaye, P.M. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J. Immunol. 158, 3813–3821 (1997).
Gomes, N.A., Gattass, C.R., Barreto-De-Souza, V., Wilson, M.E. & DosReis, G.A. TGF-β mediates CTLA-4 suppression of cellular immunity in murine kalaazar. J. Immunol. 164, 2001–2008 (2000).
Seiler, P. et al. Crucial role of marginal zone macrophages and marginal zone metalophils in the clearance of lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 27, 2626–2633 (1997).
Steinman, R.M., Pack, M. & Inaba, K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156, 25–37 (1997).
Farr, A.G. et al. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J. Exp. Med. 176, 1477–1482 (1992).
Buckley, C.D. et al. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 22, 199–204 (2001).
Gorak, P.M., Engwerda, C.R. & Kaye, P.M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28, 687–695 (1998).
Vaday, G.G. et al. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J. Leukoc. Biol. 69, 885–892 (2001).
Körner, H. et al. Distinct roles for lymphotoxin-α and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 27, 2600–2609 (1997).
Sheehan, K.C., Ruddle, N.H. & Schreiber, R.D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J. Immunol. 142, 3884–3893 (1989).
Austyn, J.M., Kupiec-Weglinski, J.W., Hankins, D.F. & Morris, P.J. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J. Exp. Med. 167, 646–651 (1988).
Gao, J.L. & Murphy, P.M. Cloning and differential tissue-specific expression of three mouse β chemokine receptor-like genes, including the gene for a functional macrophage inflammatory protein-1α receptor. J. Biol. Chem. 270, 17494–17501 (1995).
Boring, L. et al. Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein 1α receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J. Biol. Chem. 271, 7551–7558 (1996).
Manjunath, N. et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108, 871–878 (2001).
Bardi, G., Lipp, M., Baggiolini, M. & Loetscher, P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur. J. Immunol. 31, 3291–3297 (2001).
Sallusto, F. et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol 29, 1617–1625 (1999).
Engwerda, C.R., Smelt, S.C. & Kaye, P.M. An in vivo analysis of cytokine production during Leishmania donovani infection in scid mice. Exp. Parasitol. 84, 195–202 (1996).
D'Amico, G. et al. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nature Immunol. 1, 387–391 (2000).
Takayama, T. et al. Mammalian and viral IL-10 enhance C-C chemokine receptor 5 but down-regulate C-C chemokine receptor 7 expression by myeloid dendritic cells: impact on chemotactic responses and in vivo homing ability. J. Immunol. 166, 7136–7143 (2001).
O'Farrell, A.M., Liu, Y., Moore, K.W. & Mui, A.L. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 17, 1006–1018 (1998).
Dieu, M.C. et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188, 373–386 (1998).
Sozzani, S. et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 161, 1083–1086 (1998).
Kaye, P.M. Acquisition of cell-mediated immunity to Leishmania. I. Primary T-cell activation detected by IL-2 receptor expression. Immunology 61, 345–349 (1987).
Alexander, C.E., Kaye, P.M. & Engwerda, C.R. CD95 is required for the early control of parasite burden in the liver of Leishmania donovani -infected mice. Eur. J. Immunol. 31, 1199–1210 (2001).
Nickol, A.D. & Bonventre, P.F. Visceral leishmaniasis in congenic mice of susceptible and resistant phenotypes: T-lymphocyte-mediated immunosuppression. Infect. Immun. 50, 169–174 (1985).
Fu, Y.X. & Chaplin, D.D. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17, 399–433 (1999).
Weiss, L. Mechanisms of splenic control of murine malaria: cellular reactions of the spleen in lethal (strain 17XL) Plasmodium yoelii malaria in BALB/c mice, and the consequences of pre-infective splenectomy. Am. J. Trop. Med. Hyg. 41, 144–160 (1989).
Montrasio, F. et al. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288, 1257–1259 (2000).
Conlan, J.W. & North, R.J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179, 259–268 (1994).
Mercer, J.A., Wiley, C.A. & Spector, D.H. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J. Virol. 62, 987–997 (1988).
Bogdan, C. et al. Fibroblasts as host cells in latent leishmaniosis. J. Exp. Med. 191, 2121–2130 (2000).
Luster, A.D. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14, 129–135 (2002).
Cotterell, S.E., Engwerda, C.R. & Kaye, P.M. Enhanced hematopoietic activity accompanies parasite expansion in the spleen and bone marrow of mice infected with Leishmania donovani. Infect. Immun. 68, 1840–1848 (2000).
Hoffmann, K.F., Cheever, A.W & Wynn, T.A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164, 6406–6416 (2000).
Smith, D., Hansch, H., Bancroft, G. & Ehlers, S. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-α and interferon-γ. Immunology 92, 413–421 (1997).
Murray, H.W., Jungbluth, A., Ritter, E., Montelibano, C. & Marino, M.W. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect. Immun. 68, 6289–6293 (2000).
Murphy, M.L., Wille, U., Villegas, E.N., Hunter, C.A. & Farrell, J.P. IL-10 mediates susceptibility to Leishmania donovani infection. Eur. J. Immunol. 31, 2848–2856 (2001).
Basu, A., Chankrabarti, G., Saha, A. & Bandyopadhyay, S. Modulation of CD11c+ splenic dendritic cell function in murine visceral leishmaniasis: correlation with parasite replication in the spleen. Immunology 99, 305–313 (2000).
Hunter, M.G. et al. BB-10010: an active variant of human macrophage inflammatory protein-1 α with improved pharmaceutical properties. Blood 86, 4400–4408 (1995).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Meth. 223, 77–92 (1999).
Acknowledgements
We thank staff at the LSHTM Biological Services Unit for assistance in the breeding and maintenance of mouse colonies. We also thank A. Sher and J. Aliberti for comments on this manuscript. Supported by the Wellcome Trust and the British Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ato, M., Stäger, S., Engwerda, C. et al. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol 3, 1185–1191 (2002). https://doi.org/10.1038/ni861
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni861
This article is cited by
-
TIM-3 increases the abundance of type-2 dendritic cells during Leishmania donovani infection by enhancing IL-10 production via STAT3
Cell Death & Disease (2023)
-
Dendritic cell subsets in T cell programming: location dictates function
Nature Reviews Immunology (2019)
-
HIF-1α hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis
Scientific Reports (2018)
-
Regulation of immunity during visceral Leishmania infection
Parasites & Vectors (2016)
-
Expression of leukosialin (CD43) defines a major intrahepatic T cell subset associated with protective responses in visceral leishmaniasis
Parasites & Vectors (2015)