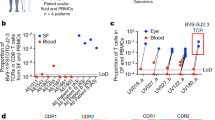
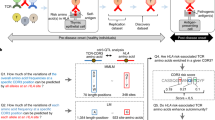
Abstract
The multiple sclerosis (MS)-associated HLA major histocompatibility complex (MHC) class II alleles DRB1*1501, DRB5*0101 and DQB1*0602 are in strong linkage disequilibrium, making it difficult to determine which is the principal MS risk gene. Here we show that together the DRB1 and DRB5 loci may influence susceptibility to MS. We demonstrate that a T cell receptor (TCR) from an MS patient recognized both a DRB1*1501-restricted myelin basic protein (MBP) and DRB5*0101-restricted Epstein-Barr virus (EBV) peptide. Crystal structure determination of the DRB5*0101-EBV peptide complex revealed a marked degree of structural equivalence to the DRB1*1501–MBP peptide complex at the surface presented for TCR recognition. This provides structural evidence for molecular mimicry involving HLA molecules. The structural details suggest an explanation for the preponderance of MHC class II associations in HLA-associated diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Change history
12 September 2002
Affiliation for Yvonne Jones was updated with note, and PDF was appended with note. Issue PDF will contain "corrected 12 September 2002 (details online). " Affiliation was formerly Department of Clinical Immunology, Rigshospitalet, DK-2200 N, Copenhagen, Denmark
References
Steinman, L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85, 299–302 (1996).
Sadovnick, A.D. et al. A population-based study of multiple sclerosis in twins: update. Ann. Neurol. 33, 281–285 (1993).
Fogdell, A., Hillert, J., Sachs, C. & Ollerup, O. The multiple sclerosis- and narcolepsy-associated HLA class II haplotype includes the DRB5*0101 allele. Tissue Antigens 46, 333–336 (1995).
Zamvil, S.S. & Steinman, L. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 8, 579–621 (1990).
Martin, R. et al. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J. Immunol. 145, 540–548 (1990).
Ota, K. et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 346 183–187 (1990).
Pette, M. et al. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc. Natl. Acad. Sci. USA 87, 7968–7972 (1990).
Li, Y., Li, H., Martin, R. & Mariuzza, R.A. Structural basis for the binding of an immunodominant peptide from myelin basic protein in different registers by two HLA-DR2 proteins. J. Mol. Biol. 304, 177–188 (2000).
Madsen, L.S. et al. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nature Genet. 23, 343–247 (1999).
Krogsgaard, M. et al. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2–MBP 85–99 complex. J. Exp. Med. 191, 1395–1412 (2000).
Sibley, W.A., Bamford, C.R. & Clark, K. Clinical viral infections and multiple sclerosis. Lancet 1, 1313–1315 (1985).
Oldstone, M.B. Molecular mimicry and autoimmune disease. Cell 50, 819–820 (1987).
Wucherpfennig, K.W. & Strominger, J.L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80, 695–705 (1995).
Nissen, P. et al. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 270, 1464–1472 (1995).
Smith, K.J., Pyrdol, J., Gauthier, L., Wiley, D.C. & Wucherpfennig, K.W. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J. Exp. Med. 188, 1511–1520 (1998).
Brown, J.H. et al. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364, 33–39 (1993).
Ghosh, P., Amaya, M., Mellins, E. & Wiley, D.C. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature 378, 457–462 (1995).
Hennecke, J., Carfi, A. & Wiley, D.C. Structure of a covalently stabilized complex of a human αβ T-cell receptor, influenza HA peptide and MHC class II molecule, HLA-DR1. EMBO J. 19, 5611–5624 (2000).
Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 19, 395–404 (1998).
Zhao, R., Loftus, D.J., Appella, E. & Collins, E.J. Structural evidence of T cell xeno-reactivity in the absence of molecular mimicry. J. Exp. Med. 189, 359–370 (1999).
Speir, J.A. et al. Structural basis of 2C TCR allorecognition of H-2Ld peptide complexes. Immunity 8, 553–562 (1998).
Basu, D., Horvath, S., Matsumoto, I., Fremont, D.H. & Allen, P.M. Molecular basis for recognition of an arthritic peptide and a foreign epitope on distinct MHC molecules by a single TCR. J. Immunol. 164, 5788–5796 (2000).
Buljevac. D. et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125, 952–960 (2002).
Panoutsakopoulou, V. et al. Analysis of the relationship between viral infection and autoimmune disease. Immunity, 15, 137–147 (2001).
Bachmaier, K. et al. Chlamydia infections and heart disease linked through antigenic mimicry. Science 283, 1335–1339 (1999).
Kouskoff, V., Signorelli, K., Benoist, C. & Mathis, D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J. Immunol. Meth. 180, 273–280 (1995).
Gauthier, L. et al. Expression and crystallization of the complex of HLA-DR2 (DRA, DRB1*1501) and an immunodominant peptide of human myelin basic protein. Proc. Natl. Acad. Sci. USA 95, 11828–11833 (1998).
Otwinowski, Z. & Minor, W. in Enzymology Vol. 276 (eds. Carter, C. W. & Sweet, R. M) 307–326 (Academic Press, New York, 1997).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994)
Brunger, A.T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta. Crystallogr A 47, 110–119 (1991).
Kraulis, P. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Esnouf, R.M. Further additions to MolScript version 1.4, including reading and contouring of electron-density maps. Acta. Crystallogr. D 55, 938–940 (1999).
Merritt E.A. & Murphy M.E.P. Raster 3d Version 2.0 - a program for photorealistic molecular graphics. Acta. Crystallogr. D 50, 869–873 (1994)
Stuart, D.I., Levine, M., Muirhead, H. & Stammers, D.K. Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 A. J. Mol. Biol. 134, 109–142 (1979).
Reid, S.W. et al. Antagonist HIV-1 Gag peptides induce structural changes in HLA- B8. J. Exp. Med. 184, 2279–2286 (1996).
Acknowledgements
We thank S. Davis, J. Boulter, C. Hourigan, N. Zaccai, G. C. Ebers and A. McMichael for useful discussions and the staff of ESRF beam line ID14 EH4 and the EMBL outstation at Grenoble for assistance with x-ray data collection. Supported by the MRC, the Karen Elise Jensen Foundation, the Danish MRC, the Danish MS Society, the Royal Society and Cancer Research UK (E. Y. J.).
*Note: In the AOP version of this article, the affiliation for E. Yvonne Jones was incorrect. It should be Division of Structural Biology, The Henry Wellcome Building for Genomic Medicine, The University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK. These errors have been corrected in the HTML version and will appear correctly in print. The PDF version available online has been appended.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lang, H., Jacobsen, H., Ikemizu, S. et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol 3, 940–943 (2002). https://doi.org/10.1038/ni835
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni835
This article is cited by
-
Non-mutational neoantigens in disease
Nature Immunology (2024)
-
Potential molecular mechanisms of chronic fatigue in long haul COVID and other viral diseases
Infectious Agents and Cancer (2023)
-
Sequence similarity between SARS-CoV-2 nucleocapsid and multiple sclerosis-associated proteins provides insight into viral neuropathogenesis following infection
Scientific Reports (2023)
-
Epstein–Barr virus as a leading cause of multiple sclerosis: mechanisms and implications
Nature Reviews Neurology (2023)
-
The contribution of thymic tolerance to central nervous system autoimmunity
Seminars in Immunopathology (2021)