Abstract
The extent of infection and rate of pathogen clearance are thought to determine both the magnitude of antigen-specific CD8+ T cell expansion and the ensuing contraction to a stable number of memory cells. We show that CD8+ T cell expansion after Listeria monocytogenes infection was primarily dependent on the initial infection dose or amount of antigen displayed, and was also influenced by the rate of pathogen clearance. However, the onset and kinetics of CD8+ T cell contraction after L. monocytogenes and lymphocytic choriomeningitis virus infections were independent of the magnitude of expansion, dose and duration of infection or amount of antigen displayed. Thus, major features of antigen-specific CD8+ T cell homeostasis, including the contraction phase of an immune response, may be programmed early after infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Sprent, J. & Surh, C.D. T cell memory. Annu. Rev. Immunol. 20, 551–579 (2002).
Harty, J.T., Tvinnereim, A.R. & White, D.W. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18, 275–308 (2000).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Wong, P. & Pamer, E.G. Cutting edge: antigen-independent CD8 T cell proliferation. J. Immunol. 166, 5864–5868 (2001).
Kaech, S.M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nature Immunol. 2, 415–422 (2001).
van Stipdonk, M. J., Lemmens, E. E. & Schoenberger, S. P. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nature Immunol. 2, 423–429 (2001).
Badovinac, V.P., Tvinnereim, A.R. & Harty, J.T. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science 290, 1354–1357 (2000).
Tvinnereim, A.R., Hamilton, S.E. & Harty, J.T. CD8+-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection. Infect. Immun. 70, 153–162 (2002).
Chen, W., Bennink, J.R. & Yewdell, J.W. Quantitating presentation of MHC class I-restricted antigens. Meth. Mol. Biol. 156, 245–254 (2001).
Harty, J.T. & Bevan, M.J. Specific immunity to Listeria monocytogenes in the absence of IFN-γ. Immunity 3, 109–117 (1995).
Gavin, M.A., Gilbert, M.J., Riddell, S.R., Greenberg, P.D. & Bevan, M.J. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J. Immunol. 151, 3971–3980 (1993).
Badovinac, V.P. & Harty, J.T. Intracellular staining for TNF and IFN-γ detects different frequencies of antigen-specific CD8+ T cells. J. Immunol. Meth. 238, 107–117 (2000).
Mercado, R. et al. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165, 6833–6839 (2000).
Smith, G.A. et al. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63, 4231–4237 (1995).
Kagi, D., Ledermann, B., Burki, K., Zinkernagel, R.M. & Hengartner, H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in imunological protection and pathogenesis. Annu. Rev. Immunol. 14, 207–232 (1996).
Murali, K.K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Lau, L.L., Jamieson, B.D., Somasundaram, T. & Ahmed, R. Cytotoxic T-cell memory without antigen. Nature 369, 648–652 (1994).
Zajac, A.J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Gallimore, A., Dumrese, T., Hengartner, H., Zinkernagel, R.M. & Rammensee, H.G. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187, 1647–1657 (1998).
Matloubian, M. et al. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 73, 2527–2536 (1999).
Busch, D.H., Pilip, I.M., Vijh, S. & Pamer, E.G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8, 353–362 (1998).
Butz, E.A. & Bevan, M.J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998).
Pope, C. et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166, 3402–349 (2001).
Marshall, D.R. et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 98, 6313–6318 (2001).
Shen, H. et al. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell 92, 535–545 (1998).
Hayflick, L. Intracellular determinants of cell aging. Mech. Age. Dev. 28, 177–185 (1984).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I peptide complexes. J. Exp. Med 187, 1383–1393 (1998).
Bach, E.A., Aguet, M. & Schreiber, R.D. The IFN γ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15, 563–591 (1997).
Pernis, A. et al. Lack of interferon γ receptor β chain and the prevention of interferon gamma signaling in TH1 cells. Science 269, 245–247 (1995).
Bach, E.A. et al. Ligand-induced autoregulation of IFN-γ receptor β chain expression in T helper cell subsets. Science 270, 1215–1218 (1995).
Tau, G.Z., Cowan, S.N., Weisburg, J., Braunstein, N.S. & Rothman, P.B. Regulation of IFN-γ signaling is essential for the cytotoxic activity of CD8+ T cells. J. Immunol. 167, 5574–5582 (2001).
Bevan, M.J. & Fink, P.J. The CD8 response on autopilot. Nature Immunol. 2, 381–382 (2001).
White, D.W. et al. Perforin-deficient CD8+ T cells: in vivo priming and antigen-specific immunity against Listeria monocytogenes. J. Immunol. 162, 980–988 (1999).
Pamer, E.G., Harty, J.T. & Bevan, M.J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature 353, 852–855 (1991).
Pamer, E.G. Direct sequence identification and kinetic analysis of an MHC class I-restricted Listeria monocytogenes CTL epitope. J. Immunol. 152, 686–694 (1994).
van der Most, R. et al. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 157, 5543–5554 (1996).
Acknowledgements
We thank E. Gutierrez for technical assistance, the NIH Tetramer Core Facility for MHC class I tetramers and S. Perlman for critical review of the manuscript. Supported by the Leukemia and Lymphoma Society (V. P. B.) and NIH grants AI42767, AI46653, AI50073 (to J. T. H) and T32A07485 (to B. B. P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Web Fig. 1.
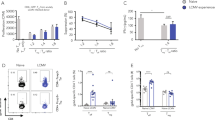
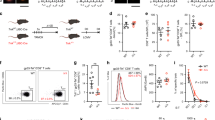
Onset of antigen-specific CD8+ T cell contraction after infection with different strains of L. monocytogenes. (a) BALB/c mice were infected with indicated doses of 10403s (virulent), DP-L1936 (plcAB-deficient), XFL303ActA (actA-deficient) and DP-L1942 (actA-deficient) and the total number of LLO + p60-specific CD8+ T cells in the spleen was determined by intracellular cytokine staining for IFN-γ. Mice that contained viable bacteria in the liver at day 7 after infection are marked (*). Data represent mean ± s.d. of three mice per group per time point. (b) Contraction of antigen-specific CD8+ T cells in the spleen at the indicated time points after L. monocytogenes infection was normalized to the peak of the primary response (day 7); presented as 100%. (JPG 42 kb)
Web Fig. 2.
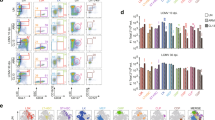
Visualizing primary and secondary CD8+ T cell responses in the same host. (a) BALB/c Thy1.2 mice were infected with LCMV-Armstrong. One-hundred days later splenocytes (1 × 107) from LCMV memory mice were transferred into naïve BALB/c Thy1.1 mice. Two days after transfer recipient BALB/c Thy1.1 mice were infected with 1 or 0.1 LD50 of XFL303. (b) Frequency of Thy1.2 NP(118–126)-specific CD8+ T cells before and after transfer into naïve Thy1.1 mice. (c) Total number of CD8+ Thy1.2+ and NP(118–126)-specific CD8+ Thy1.2+ cells before and after the transfer. Numbers represent the percentage of transferred CD8+ and NP(118–126)-specific cells in the spleens of mice that received 107 donor cells. (JPG 36 kb)
Rights and permissions
About this article
Cite this article
Badovinac, V., Porter, B. & Harty, J. Programmed contraction of CD8+ T cells after infection. Nat Immunol 3, 619–626 (2002). https://doi.org/10.1038/ni804
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni804
This article is cited by
-
The effect of dosage on the protective efficacy of whole-sporozoite formulations for immunization against malaria
npj Vaccines (2023)
-
A novel oncolytic virus engineered with PD-L1 scFv effectively inhibits tumor growth in a mouse model
Cellular & Molecular Immunology (2019)
-
Dependence of CD8 T Cell Response upon Antigen Load During Primary Infection
Bulletin of Mathematical Biology (2019)
-
A functional subset of CD8+ T cells during chronic exhaustion is defined by SIRPα expression
Nature Communications (2019)
-
Memory responses of innate lymphocytes and parallels with T cells
Seminars in Immunopathology (2018)