Abstract
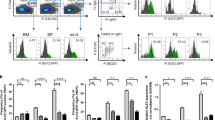
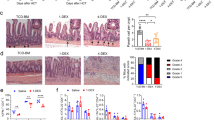
Peyer's patches (PPs) and/or mesenteric lymph nodes (MLNs) are thought to be essential for immunoglobulin A (IgA) production. We found that the severe IgA deficiency in lymphotoxin-deficient (LT−/−) mice could be fully reversed by reconstitution with LT-expressing bone marrow, despite the absence of both LNs and PPs. The number of IgA precursors from LT−/− mice was not reduced, and they were able to migrate into the lamina propria (LP) of wild-type mice but not of LTβR−/− mice. Consistently, lymphoid tissue chemokines and adhesion molecules were reduced within the LP of LTα−/− and LTβR−/− mice. IgA deficiency in LTα−/− mice was reversed by the transplantation of a segment of RAG-1 (recombination-activating gene 1)–deficient intestine, which confirmed the dispensability of the MLNs and PPs and the sufficiency of the LT-mediated gut microenvironment for IgA production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
20 May 2002
Online figure was updated, PDF was ammended with note. Issue PDF contains "corrected 20 May 2002 (details online)" line.
References
Brandtzaeg, P. et al. The B-cell system of human mucosae and exocrine glands. Immunol. Rev. 171, 45–87 (1999).
Weiner, H. et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu. Rev. Immunol. 12, 809–837 (1994).
Staats, H.F. et al. Mucosal immunity to infection with implications for vaccine development. Curr. Opin. Immunol. 6, 572–583 (1994).
Burrows, P.D. & Cooper, M.D. IgA deficiency. Adv. Immunol. 65, 245–276 (1997).
Macpherson, A.J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000).
Sandler, S.G., Mallory, D., Malamut, D. & Eckrich, R. IgA anaphylactic transfusion reactions. Transfus. Med. Rev. 9, 1–8 (1995).
Craig, S.W. & Cebra, J.J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J. Exp. Med. 134, 188–200 (1971).
Husband, A.J. & Gowans, J.L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J. Exp. Med. 148, 1146–1160 (1978).
Yamamoto, M. et al. Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J. Immunol. 164, 5184–5191 (2000).
Spahn, T.W. et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur. J. Immunol. 31, 1278–1287 (2001).
Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & Honjo, T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413, 639–643 (2001).
Brandtzaeg, P., Baekkevold, E.S. & Morton, H.C. From B to A the mucosal way. Nature Immunol. 2, 1093–1094 (2001).
Berland, R. & Wortis, H.H. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20, 253–300 (2002).
Mowat, A.M. & Viney, J.L. The anatomical basis of intestinal immunity. Immunol. Rev. 156, 145–166 (1997).
Griebel, P.J. & Hein, W.R. Expanding the role of Peyer's patches in B-cell ontogeny. Immunol. Today 17, 30–39 (1996).
Husband, A.J., Bao, S. & Beagley, K.W. Analysis of the mucosal microenvironment: factors determining successful responses to mucosal vaccines. Vet. Immunol. Immunopathol. 72, 135–142 (1999).
Fujihashi, K. et al. Peyer's patches are required for oral tolerance to proteins. Proc. Natl. Acad. Sci. USA 98, 3310–3315 (2001).
Fagarasan, S. et al. Mechanism of B1 cell differentiation and migration in GALT. Curr. Top. Microbiol. Immunol. 252, 221–229 (2000).
Kroese, F.G., de Waard, R. & Bos, N. A. B-1 cells and their reactivity with the murine intestinal microflora. Semin. Immunol. 8, 11–18 (1996).
Auci, D.L., Chice, S.M., Heusser, C., Athanassiades, T.J. & Durkin, H.G. Origin and fate of IgE-bearing lymphocytes. II. Gut-associated lymphoid tissue as sites of first appearance of IgE-bearing B lymphocytes and hapten-specific IgE antibody-forming cells in mice immunized with benzylpenicilloyl-keyhole limpet hemocyanin by various routes: relation to asialo GM1 ganglioside+ cells and IgE/CD23 immune complexes. J. Immunol. 149, 2241–2248 (1992).
Fagarasan, S. et al. Alymphoplasia (aly)-type nuclear factor κB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J. Exp. Med. 191, 1477–1486 (2000).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Banks, T.A. et al. Lymphotoxin-α-deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 155, 1685–1693 (1995).
Fu, Y.X. & Chaplin, D.D. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17, 399–433 (1999).
Davis, I.A., Knight, K.A. & Rouse, B.T. The spleen and organized lymph nodes are not essential for the development of gut-induced mucosal immune responses in lymphotoxin- alpha deficient mice. Clin. Immunol. Immunopathol. 89, 150–159 (1998).
Ware, C., VanArsdale, T., Crowe, P. & Browning, J. The ligands and receptors of the lymphotoxin system. Curr. Top. Microbiol. Immunol. 198, 175–218 (1995).
Pasparakis, M. et al. Peyers patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl. Acad. Sci. USA 94, 6319–6323 (1997).
Ryffel, B., Le Hir, M., Muller, M. & Eugster, H.P. Correction of the TNF-LTα-deficient phenotype by bone marrow transplantation. Dev. Immunol. 6, 253–260 (1998).
Davis, I.A. & Rouse, B.T. Immune responsiveness of lymphotoxin-α-deficient mice: two reconstitution models. Cell. Immunol. 189, 116–124 (1998).
Ansel, K.M., Harris, R.B. & Cyster, J.G. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16, 67–76 (2002).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Ngo, V.N. et al. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 (1999).
Gunn, M.D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Kunkel, E.J. & Butcher, E.C. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1–4 (2002).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Mackay, F., Majeau, G.R., Lawton, P., Hochman, P.S. & Browning, J.L. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur. J. Immunol. 27, 2033–2042 (1997).
Macpherson, A.J. et al. IgA production without μ or δ chain expression in developing B cells. Nature Immunol. 2, 625–631 (2001).
Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M.H. & Pfeffer, K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
Rennert, P.D., Browning, J.L., Mebius, R., Mackay, F. & Hochman, P.S. Surface lymphotoxinα/β complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 184, 1999–2006 (1996).
Fu, Y.X. et al. Lymphotoxin-α (LTα) supports development of splenic follicular structure that is required for IgG responses. J. Exp. Med. 185, 2111–2120 (1997).
Wang, J. et al. The regulation of T cell homeostasis and autoimmunity by T cell derived LIGHT. J. Clin. Invest. 108,1771–1780 (2001).
Lefrancois, L. & Lycke, N. in Current Protocol Immunology Vol. 1 (ed. Coico, R.) 3.19.1–16 (John Wiley & Sons, New York, 1996).
Guo, Z. et al. Cutting edge: membrane lymphotoxin regulates CD8+ T cell-mediated intestinal allograft rejection. J. Immunol. 167, 4796–4800 (2001).
Acknowledgements
We thank the National Cell Culture Center for the LTβR-Ig. Supported in part by NIH grants (HD-37104 and DK-58897) and Biogen. LTβR−/− mice were kindly provided by K. Pfeffer (Technical University of Munich, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kang, HS., Chin, R., Wang, Y. et al. Signaling via LTβR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol 3, 576–582 (2002). https://doi.org/10.1038/ni795
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni795
This article is cited by
-
Fructose malabsorption in ChREBP-deficient mice disrupts the small intestine immune microenvironment and leads to diarrhea-dominant bowel habit changes
Inflammation Research (2023)
-
Neonatal LTβR signaling is required for the accumulation of eosinophils in the inflamed adult mesenteric lymph node
Mucosal Immunology (2022)
-
IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production
Mucosal Immunology (2015)
-
Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy
Nature (2015)
-
Transcriptional fingerprints of antigen-presenting cell subsets in the human vaginal mucosa and skin reflect tissue-specific immune microenvironments
Genome Medicine (2014)