Abstract
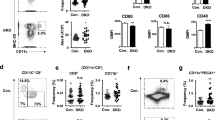
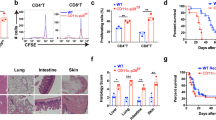
The function of interleukin 15 (IL-15) in unmethylated CpG oligodeoxynucleotide (CpG)–induced immune responses remains unknown. Here, in response to CpG, both wild-type and natural killer cell–depleted mice produced IL-12 and became resistant to a lethal dose of Listeria monocytogenes. In contrast, CpG-treated IL-15-deficient mice produced little IL-12 and succumbed to L. monocytogenes. CpG-stimulated conventional dendritic cells (cDCs) were the main producers of both IL-15 and IL-12, but cDCs did not produce IL-12 in the absence of plasmacytoid DCs (pDCs). The cDC-derived IL-15 induced CD40 expression by cDCs. Interaction between CD40 on cDCs and CD40 ligand on pDCs led to IL-12 production by cDCs. Thus, IL-15-dependent crosstalk between cDCs and pDCs is essential for CpG-induced immune activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20, 709–760 (2002).
Wagner, H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr. Opin. Microbiol. 5, 62–69 (2002).
Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 4, 249–258 (2004).
Wesche, H., Henzel, W.J., Shillinglaw, W., Li, S. & Cao, Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 (1997).
Cao, Z., Xiong, J., Takeuchi, M., Kurama, T. & Goeddel, D.V. TRAF6 is a signal transducer for interleukin-1. Nature 383, 443–446 (1996).
Shirakabe, K. et al. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J. Biol. Chem. 272, 8141–8144 (1997).
DiDonato, J.A., Hayakawa, M., Rothwarf, D.M., Zandi, E. & Karin, M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388, 548–554 (1997).
Waldmann, T.A. & Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17, 19–49 (1999).
Ogasawara, K. et al. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature 391, 700–703 (1998).
Ohteki, T. et al. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-α/β+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 187, 967–972 (1998).
Kennedy, M.K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Lodolce, J.P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Ohteki, T., Suzue, K., Maki, C., Ota, T. & Koyasu, S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2, 1138–1143 (2001).
Krieg, A.M., Love-Homan, L., Yi, A.-K. & Harty, J.T. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161, 2428–2434 (1998).
Elkins, K.L., Rhinehart-Jones, T.R., Stibitz, S., Conover, J.S. & Klinman, D.M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162, 2291–2298 (1999).
Hemmi, H. et al. A toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Takeshita, F. et al. Role of toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167, 3555–3558 (2001).
Bauer, S. et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98, 9237–9242 (2001).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Colonna, M., Trinchieri, G. & Liu, Y.-J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 (2004).
Barchet, W., Cella, M. & Colonna, M. Plasmacytoid dendritic cells–-virus experts of innate immunity. Semin. Immunol. 17, 253–261 (2005).
Krug, A. et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21, 107–119 (2004).
Barchet, W. et al. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. 35, 236–242 (2005).
Yoneyama, H. et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202, 425–435 (2005).
Lodolce, J.P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Dubois, S., Mariner, J., Waldmann, T.A. & Tagaya, Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 (2002).
Suzuki, H. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476 (1995).
Kawabe, T. et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1, 167–178 (1994).
Xu, J. et al. Mice deficient for the CD40 ligand. Immunity 1, 423–431 (1994).
Yamamoto, S. et al. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J. Immunol. 148, 4072–4076 (1992).
Krieg, A.M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–548 (1995).
Klinman, D.M., Yi, A., Beaucage, S.L., Conover, J. & Krieg, A.M. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNγ. Proc. Natl. Acad. Sci. USA 93, 2879–2883 (1996).
Dittmer, U. & Olbrich, A.R. Treatment of infectious diseases with immunostimulatory oligodeoxynucleotides containing CpG motifs. Curr. Opin. Microbiol. 6, 472–477 (2003).
Finlay, B.B. & Hancock, R.E. Can innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2, 497–504 (2004).
Jahrsdorfer, B. & Weiner, G.J. CpG oligodeoxynucleotides for immune stimulation in cancer immunotherapy. Curr. Opin. Investig. Drugs 4, 686–690 (2003).
Carpentier, A.F., Auf, G. & Delattre, J.Y. CpG oligodeoxynucleotides for cancer immunotherapy, review of the literature and potential applications in malignant glioma. Front. Biosci. 8, 115–127 (2003).
Kline, J.N. et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 160, 2555–2559 (1998).
Weiss, R. et al. Design of protective and therapeutic DNA vaccines for the treatment of allergic diseases. Curr. Drug Targets Inflamm. Allergy 4, 585–597 (2005).
Krug, A. et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31, 3026–3037 (2001).
Schulz, O. et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13, 453–462 (2000).
Mackey, M.F. et al. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J. Immunol. 161, 2094–2098 (1998).
Fontana, S. et al. Functional defects of dendritic cells in patients with CD40 deficiency. Blood 102, 4099–4106 (2003).
Ridge, J.P., Di, R.F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ helper cell and T-killer cell. Nature 393, 474–478 (1998).
Bennett, S.R. et al. Help for cytotoxic T-cell responses is mediated by CD40 signalling. Nature 393, 478–480 (1998).
Schoenberger, S.P., Toes, R.E.M., van der Voort, E.I.H., Offringa, R. & Melief, J.M. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 393, 480–483 (1998).
Smith, C.M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol. 5, 1143–1148 (2004).
Buller, R.M., Holmes, K.L., Hugin, A., Frederickson, T.N. & Morse, H.C. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature 328, 77–79 (1987).
Rahemtulla, A. et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353, 180–184 (1991).
Ishii, K.J. et al. CpG-activated Thy1.2+ dendritic cells protect against lethal Listeria monocytogenes infection. Eur. J. Immunol. 35, 2397–2405 (2005).
Marzo, A.L. et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 173, 969–975 (2004).
Nguyen, K.B. et al. Coordinated and distinct roles for IFN-αβ, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169, 4279–4287 (2002).
Krug, A. et al. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103, 1433–1437 (2004).
Hochrein, H. et al. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and independent pathways. Proc. Natl. Acad. Sci. USA 101, 11416–11421 (2004).
Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 (2003).
Acknowledgements
We thank M. Shibata, N. Kakizaki and K. Onodera for animal care; K. Yamashita and Y. Abe for experimental support; K. Hasegawa for discussions; S. Jung (Weizmann Institute of Science, Rehovot, Israel) for DTR-GFP mice; T.W. Mak (Campbell Family Institute, Toronto, Canada) for B6 Il2rb−/− mice; H. Kikutani (Osaka University, Suita, Japan) for B6 Cd40−/− mice; R. Abe (Tokyo University of Science, Noda, Japan) for B6 Cd40lg−/− mice; and H. Yagita (Juntendo University, Tokyo, Japan) for mAb MR1. Supported by the Toray Science Foundation (T.O.), the Takeda Science Foundation (T.O.), the Novartis Foundation for the Promotion of Science (T.O.), the Sankyo Foundation for Life Science (T.O.), a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan (16017212 and 16043204 to T.O.), a Grant-in-Aid for Creative Scientific Research by the Japan Society for the Promotion of Science (13GS0015) and the Japan Society for the Promotion of Science for Young Scientists (H.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
The kinetic analysis of serum IFN-γ production after CpG injection. (PDF 250 kb)
Supplementary Fig. 2
The kinetic analysis of serum IL-12 p70 production after CpG injection. (PDF 247 kb)
Supplementary Figure 3
A system of mixed BM-chimeras. (PDF 520 kb)
Rights and permissions
About this article
Cite this article
Kuwajima, S., Sato, T., Ishida, K. et al. Interleukin 15–dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat Immunol 7, 740–746 (2006). https://doi.org/10.1038/ni1348
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1348
This article is cited by
-
Fms-like tyrosine kinase-3 ligand increases resistance to burn wound infection through effects on plasmacytoid dendritic cells
BMC Immunology (2017)
-
Plasmacytoid dendritic cells orchestrate TLR7-mediated innate and adaptive immunity for the initiation of autoimmune inflammation
Scientific Reports (2016)
-
Local Immune Stimulation by Intravesical Instillation of Baculovirus to Enable Bladder Cancer Therapy
Scientific Reports (2016)
-
Splenic CD11c(+) cells derived from semi-immune mice protect naïve mice against experimental cerebral malaria
Malaria Journal (2015)
-
Slc15a4 function is required for intact class switch recombination to IgG2c in response to TLR9 stimulation
Immunology & Cell Biology (2015)