Abstract
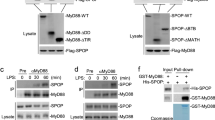
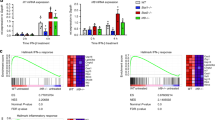
The MyD88 adaptor protein is critical in Toll-like receptor and interleukin 1 receptor (IL-1R) signaling, but has not been linked to interferon-γ receptor (IFN-γR) signaling. Here we demonstrate that MyD88 increased the half-life but not the synthesis of IFN-γ-induced mRNA transcripts encoding tumor necrosis factor and IFN-γ-inducible protein 10. IFN-γ stimulation triggered a physical association between the IFN-γR1 and MyD88. Transcript stabilization required activation of mixed-lineage kinase 3 and p38 mitogen-activated protein kinase and the presence of an adenine-uridine–rich element in the transcript's 3′ untranslated region. These results demonstrate a MyD88-dependent post-transcriptional mechanism through which IFN-γ can enhance the expression of genes encoding proinflammatory molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nathan, C.F. Secretory products of macrophages. J. Clin. Invest. 79, 319–326 (1987).
Han, J. & Ulevitch, R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 6, 1198–1205 (2005).
Wilusz, C.J., Wormington, M. & Peltz, S.W. The cap-to-tail guide to mRNA turnover. Nat. Rev. Mol. Cell Biol. 2, 237–246 (2001).
Lam, L.T. et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2, 1–11 (2001).
Grigull, J., Mnaimneh, S., Pootoolal, J., Robinson, M.D. & Hughes, T.R. Genome-wide analysis of mRNA stability using transcription inhibitors and microarrays reveals posttranscriptional control of ribosome biogenesis factors. Mol. Cell. Biol. 24, 5534–5547 (2004).
Shaw, G. & Kamen, R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46, 659–667 (1986).
Treisman, R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5′ element and c-fos 3′ sequences. Cell 42, 889–902 (1985).
Gingerich, T.J., Feige, J.J. & LaMarre, J. AU-rich elements and the control of gene expression through regulated mRNA stability. Anim. Health Res. Rev. 5, 49–63 (2004).
Datta, S., Novotny, M., Li, X., Tebo, J. & Hamilton, T.A. Toll IL-1 receptors differ in their ability to promote the stabilization of adenosine and uridine-rich elements containing mRNA. J. Immunol. 173, 2755–2761 (2004).
Bakheet, T., Frevel, M., Williams, B.R., Greer, W. & Khabar, K.S. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 29, 246–254 (2001).
Brennan, C.M. & Steitz, J.A. HuR and mRNA stability. Cell. Mol. Life Sci. 58, 266–277 (2001).
Lai, W.S. et al. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor-α mRNA. Mol. Cell. Biol. 19, 4311–4323 (1999).
Jing, Q. et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120, 623–634 (2005).
Carballo, E., Lai, W.S. & Blackshear, P.J. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 281, 1001–1005 (1998).
Tchen, C.R., Brook, M., Saklatvala, J. & Clark, A.R. The stability of tristetraprolin mRNA is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J. Biol. Chem. 279, 32393–32400 (2004).
Gouble, A. et al. A new player in oncogenesis: AUF1/hnRNPD overexpression leads to tumorigenesis in transgenic mice. Cancer Res. 62, 1489–1495 (2002).
Taylor, G.A. et al. A pathogenetic role for TNF-α in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4, 445–454 (1996).
Kontoyiannis, D., Pasparakis, M., Pizarro, T.T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Mahtani, K.R. et al. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor-α mRNA stability. Mol. Cell. Biol. 21, 6461–6469 (2001).
Frevel, M.A. et al. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell. Biol. 23, 425–436 (2003).
Winzen, R. et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18, 4969–4980 (1999).
Neininger, A. et al. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J. Biol. Chem. 277, 3065–3068 (2002).
Stoecklin, G. et al. MK2-induced tristetraprolin:14–3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 23, 1313–1324 (2004).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Darnell, J.E., Jr., Kerr, I.M. & Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994).
Shi, S. et al. MyD88 primes macrophages for full-scale activation by interferon-γ yet mediates few responses to Mycobacterium tuberculosis. J. Exp. Med. 198, 987–997 (2003).
Meraz, M.A. et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84, 431–442 (1996).
Shuai, K., Schindler, C., Prezioso, V.R. & Darnell, J.E., Jr. Activation of transcription by IFN-γ: tyrosine phosphorylation of a 91-kD DNA binding protein. Science 258, 1808–1812 (1992).
Hoebe, K. et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424, 743–748 (2003).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Ichijo, H. et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90–94 (1997).
Teramoto, H. et al. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J. Biol. Chem. 271, 27225–27228 (1996).
Sato, S. et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171, 4304–4310 (2003).
Wesche, H., Henzel, W.J., Shillinglaw, W., Li, S. & Cao, Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 (1997).
Medzhitov, R. et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2, 253–258 (1998).
Morrison, D.K. & Davis, R.J. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19, 91–118 (2003).
Whitmarsh, A.J., Cavanagh, J., Tournier, C., Yasuda, J. & Davis, R.J. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 281, 1671–1674 (1998).
Goh, K.C., Haque, S.J. & Williams, B.R. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 18, 5601–5608 (1999).
Janssens, S. & Beyaert, R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11, 293–302 (2003).
Gallo, K.A. & Johnson, G.L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 3, 663–672 (2002).
Roy, S.K. et al. A role for mixed lineage kinases in regulating transcription factor CCAAT/enhancer-binding protein-β-dependent gene expression in response to interferon-γ. J. Biol. Chem. 280, 24462–24471 (2005).
Jefferies, C.A. et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor κB activation by Toll-like receptor 4. J. Biol. Chem. 278, 26258–26264 (2003).
Ojaniemi, M. et al. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur. J. Immunol. 33, 597–605 (2003).
Honda, K. et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 101, 15416–15421 (2004).
Wang, T.Y., Gu, S., Ronni, T., Du, Y. & Chen, X. In vivo dual-tagging proteome approach in studying signaling pathways in immune response. J. Proteome Res. 4, 941–949 (2005).
Kawai, T. et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167, 5887–5894 (2001).
Yamamoto, M. et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 (2002).
Hoshino, K., Kaisho, T., Iwabe, T., Takeuchi, O. & Akira, S. Differential involvement of IFN-β in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 14, 1225–1231 (2002).
Verma, A. et al. Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-β on normal hematopoiesis. J. Biol. Chem. 277, 7726–7735 (2002).
Scanga, C.A. et al. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 72, 2400–2404 (2004).
Fremond, C.M. et al. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J. Clin. Invest. 114, 1790–1799 (2004).
Shi, S. et al. Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-αβ receptor and STAT1. J. Immunol. 175, 3318–3328 (2005).
Mogues, T., Goodrich, M.E., Ryan, L., LaCourse, R. & North, R.J. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193, 271–280 (2001).
Williams, B.R. Signal integration via PKR. Sci. STKE 3 July 2001 (10.1126/stke.2001.89.re2).
Jin, F.Y., Nathan, C., Radzioch, D. & Ding, A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 88, 417–426 (1997).
Ehrt, S. et al. Reprogramming of the macrophage transcriptome in response to interferon-γ and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194, 1123–1140 (2001).
Acknowledgements
We thank H. Yu for technical assistance, and C. Nathan and K. Rhee for critical reading of the manuscript. Supported by the National Institutes of Health (AI30165 and GM61710 to A.D.) and the William Randolph Hearst Foundation (to The Department of Microbiology and Immunology).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sun, D., Ding, A. MyD88-mediated stabilization of interferon-γ-induced cytokine and chemokine mRNA. Nat Immunol 7, 375–381 (2006). https://doi.org/10.1038/ni1308
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1308
This article is cited by
-
Inflammatory gene silencing in activated monocytes by a cholesterol tagged-miRNA/siRNA: a novel approach to ameliorate diabetes induced inflammation
Cell and Tissue Research (2022)
-
MyD88 and beyond: a perspective on MyD88-targeted therapeutic approach for modulation of host immunity
Immunologic Research (2021)
-
Intracerebral overexpression of miR-669c is protective in mouse ischemic stroke model by targeting MyD88 and inducing alternative microglial/macrophage activation
Journal of Neuroinflammation (2020)
-
Functional analysis and clinical significance of sodium iodide symporter expression in gastric cancer
Gastric Cancer (2019)
-
Small Molecule Analogues of the parasitic worm product ES-62 interact with the TIR domain of MyD88 to inhibit pro-inflammatory signalling
Scientific Reports (2018)