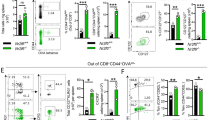
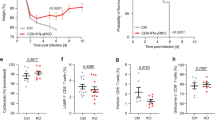
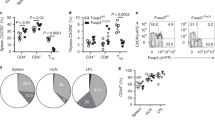
Abstract
Listeria monocytogenes infection generates major histocompatibility complex (MHC) class Ia–restricted and MHC class Ib-(H2-M3)–restricted effector and memory CD8+ T cells. However, only MHC class Ia–restricted memory cells expand after rechallenge, and it is unknown if MHC class Ib–restricted memory CD8+ T cells generated by vaccination are protective. We show here that H2-M3-restricted memory CD8+ T cells were capable of secondary expansion but, in contrast to primary H2-M3-restricted effector cells, failed to provide protective immunity. In lm-immune mice, MHC class Ia–restricted memory CD8+ T cells prevented the expansion of H2-M3-restricted memory T cell populations by limiting dendritic cell antigen presentation. Thus, protective immunity by H2-M3-resricted T cells is limited to primary infection, indicating that memory MHC class Ia–restricted T cells prevent nonessential immune responses during secondary infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lindahl, K.F. et al. H2-M3, a full-service class Ib histocompatibility antigen. Annu. Rev. Immunol. 15, 851–879 (1997).
Lenz, L.L., Dere, B. & Bevan, M.J. Identification of an H2-M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity 5, 63–72 (1996).
Shawar, S.M., Cook, R.G., Rodgers, J.R. & Rich, R.R. Specialized functions of MHC class I molecules. I. An N-formyl peptide receptor is required for construction of the class I antigen Mta. J. Exp. Med. 171, 897–912 (1990).
Shawar, S.M., Vyas, J.M., Rodgers, J.R., Cook, R.G. & Rich, R.R. Specialized functions of major histocompatibility complex class I molecules. II. Hmt binds N-formylated peptides of mitochondrial and prokaryotic origin. J. Exp. Med. 174, 941–944 (1991).
Wang, C.R., Loveland, B.E. & Lindahl, K.F. H-2M3 encodes the MHC class I molecule presenting the maternally transmitted antigen of the mouse. Cell 66, 335–345 (1991).
Kerksiek, K.M., Busch, D.H. & Pamer, E.G. Variable immunodominance hierarchies for H2-M3-restricted N-formyl peptides following bacterial infection. J. Immunol. 166, 1132–1140 (2001).
Chun, T. et al. Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis. J. Exp. Med. 193, 1213–1220 (2001).
Berg, R.E. et al. Positive selection of an H2-M3 restricted T cell receptor. Immunity 11, 33–43 (1999).
Chiu, N.M. et al. The selection of M3-restricted T cells is dependent on M3 expression and presentation of N-formylated peptides in the thymus. J. Exp. Med. 190, 1869–1878 (1999).
Urdahl, K.B., Sun, J.C. & Bevan, M.J. Positive selection of MHC class Ib-restricted CD8+ T cells on hematopoietic cells. Nat. Immunol. 3, 772–779 (2002).
Busch, D., Pilip, I., Vijh, S. & Pamer, E. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8, 353–362 (1998).
Kerksiek, K.M., Busch, D.H., Pilip, I.M., Allen, S.E. & Pamer, E.G. H2-M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J. Exp. Med. 190, 195–204 (1999).
Kerksiek, K.M., Ploss, A., Leiner, I., Busch, D.H. & Pamer, E.G. H2-M3-restricted memory T cells: persistence and activation without expansion. J. Immunol. 170, 1862–1869 (2003).
Badovinac, V.P., Porter, B.B. & Harty, J.T. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3, 619–626 (2002).
Kaufmann, S.H., Rodewald, H.R., Hug, E. & De Libero, G. Cloned Listeria monocytogenes specific non-MHC-restricted Lyt-2+ T cells with cytolytic and protective activity. J. Immunol. 140, 3173–3179 (1988).
Lukacs, K. & Kurlander, R.J. MHC-unrestricted transfer of anti-listerial immunity by freshly isolated immune CD8 spleen cells. J. Immunol. 143, 3731–3736 (1989).
Seaman, M.S., Wang, C.R. & Forman, J. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J. Immunol. 165, 5192–5201 (2000).
D'Orazio, S.E.F., Halme, D.G., Ploegh, H.L. & Starnbach, M.N. Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection. J. Immunol. 171, 291–298 (2003).
Bouwer, H.G.A., Seaman, M.S., Forman, J. & Hinrichs, D.J. MHC class Ib-restricted cells contribute to anti-listerial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J. Immunol. 159, 2795–2801 (1997).
Seaman, M.S., Perarnau, B., Lindahl, K.F., Lemonnier, F.A. & Forman, J. Response to Listeria monocytogenes in mice lacking MHC class Ia molecules. J. Immunol. 162, 5429–5436 (1999).
Rolph, M.S. & Kaufmann, S.H. Partially TAP-independent protection against Listeria monocytogenes by H2-M3-restricted CD8+ T cells. J. Immunol. 165, 4575–4580 (2000).
Hamilton, S.E. & Harty, J.T. Quantitation of CD8+ T cell expansion, memory, and protective immunity after immunization with peptide-coated dendritic cells. J. Immunol. 169, 4936–4944 (2002).
Harty, J.T., Tvinnereim, A.R. & White, D.W. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18, 275–308 (2000).
Jensen, E.R. et al. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect. Immun. 66, 4143–4150 (1998).
Barber, D.L., Wherry, E.J. & Ahmed, R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171, 27–31 (2003).
Byers, A.M., Kemball, C.C., Moser, J.M. & Lukacher, A.E. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J. Immunol. 171, 17–21 (2003).
White, D.W. & Harty, J.T. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-γ but requires TNF-α. J. Immunol. 160, 898–905 (1998).
Russell, J.H. & Ley, T.J. Lymphocyte-mediated cytotoxicity. Ann. Rev. Immunol. 20, 323–370 (2002).
Kagi, D., Ledermann, B., Buerki, K., Hengartner, H. & Zinkernagel, R.M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur. J. Immunol. 24, 3068–3072 (1994).
San Mateo, L.R., Chua, M.M., Weiss, S.R. & Shen, H. Perforin-mediated CTL cytolysis counteracts direct cell-cell spread of Listeria monocytogenes. J. Immunol. 169, 5202–5208 (2002).
Messingham, K.A.N., Badovinac, V.P. & Harty, J.T. Deficient anti-listerial immunity in the absence of perforin can be restored by increasing memory CD8+ T cell numbers. J. Immunol. 171, 4254–4262 (2003).
Tawab, A., Fields, J., Chao, E. & Kurlander, R.J. Recombinant lemA without adjuvant induces extensive expansion of H2-M3-restricted CD8 effectors, which can suppress primary listeriosis in mice. Internat. Immunol. 14, 225–232 (2001).
Walsh, C.M. et al. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91, 10854–10858 (1994).
Kaech, S.M., Wherry, E.J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 (2002).
Shen, H. et al. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell 92, 535–545 (1998).
Wong, P. & Pamer, E.G. Feedback regulation of pathogen-specific T cell priming. Immunity 18, 499–511 (2003).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Butz, E.A. & Bevan, M.J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998).
Kedl, R.M. et al. T cells compete for access to antigen-bearing antigen-presenting cells. J. Exp. Med. 192, 1105–1113 (2000).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Migueles, S.A. et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3, 1061 (2002).
Lenz, L.L. & Bevan, M.J. CTL responses to H2-M3-restricted Listeria epitopes. Immunol. Rev. 158, 115–121 (1997).
Urdahl, K.B., Liggitt, D. & Bevan, M.J. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb−/−Db−/− mice, but provide minimal protection. J. Immunol. 170, 1987–1994 (2003).
Crowe, S.R. et al. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 198, 399–410 (2003).
White, D.W. et al. Perforin-deficient CD8+ T cells: in vivo priming and antigen-specific immunity against Listeria monocytogenes. J. Immunol. 162, 980–988 (1999).
Bishop, D.K. & Hinrichs, D.J. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139, 2005–2009 (1987).
Harty, J.T. & Bevan, M.J. Specific immunity to Listeria monocytogenes in the absence of IFN-γ. Immunity 3, 109–117 (1995).
Badovinac, V.P., Tvinnereim, A.R. & Harty, J.T. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science 290, 1354–1358 (2000).
Badovinac, V.P., Hamilton, S.E. & Harty, J.T. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18, 463–474 (2003).
Acknowledgements
We thank K. Rensberger and R. Podyminogin for technical assistance; E. Pamer (Memorial Sloan Kettering, New York, New York) for TCR-transgenic mice; C.-R. Wang (University of Chicago, Chicago, Illinois) for H2-M3-specific antibody; and S. Perlman (University of Iowa, Iowa City, Iowa) for critical review of the manuscript. We also thank the National Institute of Allergy and Infectious Disease tetramer core facility for reagents. Supported by National Institutes of Health grants AI42767, AI46653, AI50073 (J.T.H.), T32AI07511 (S.E.H.), T32AI07485 (B.B.P.) and T32AI07260 (K.A.N.M.), and a Leukemia and Lymphoma Society Fellow Grant (V.P.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hamilton, S., Porter, B., Messingham, K. et al. MHC class Ia–restricted memory T cells inhibit expansion of a nonprotective MHC class Ib (H2-M3)–restricted memory response. Nat Immunol 5, 159–168 (2004). https://doi.org/10.1038/ni1026
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1026
This article is cited by
-
The role of MHC class Ib-restricted T cells during infection
Immunogenetics (2016)
-
MHC class II‐dependent T–T interactions create a diverse, functional and immunoregulatory reaction circle
Immunology & Cell Biology (2009)
-
MHC class Ib molecules bridge innate and acquired immunity
Nature Reviews Immunology (2005)
-
Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination
Nature Medicine (2005)
-
Immune responses to Listeria monocytogenes
Nature Reviews Immunology (2004)