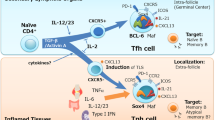
Abstract
CD4+ effector T cells have been categorized into two subsets: T helper type 1 (TH1) and TH2. Another subset of T cells that produce interleukin 17 (IL-17; 'TH-17 cells') has been identified that is highly proinflammatory and induces severe autoimmunity. Whereas IL-23 serves to expand previously differentiated TH-17 cell populations, IL-6 and transforming growth factor-β (TGF-β) induce the differentiation of TH-17 cells from naive precursors. These data suggest a dichotomy between CD4+ regulatory T cells positive for the transcription factor Foxp3 and TH-17 cells: TGF-β induces Foxp3 and generates induced regulatory T cells, whereas IL-6 inhibits TGF-β-driven Foxp3 expression and together with TGF-β induces TH-17 cells. Emerging data regarding TH-17 cells suggest a very important function for this T cell subset in immunity and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mosmann, T.R. & Coffman, R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Abbas, A.K., Murphy, K.M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
Shevach, E.M. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25, 195–201 (2006).
Sakaguchi, S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101, 455–458 (2000).
Faria, A.M. & Weiner, H.L. Oral tolerance and TGF-beta-producing cells. Inflamm. Allergy Drug Targets 5, 179–190 (2006).
Wan, Y.Y. & Flavell, R.A. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol. Rev. 212, 114–130 (2006).
Willenborg, D.O., Fordham, S., Bernard, C.C., Cowden, W.B. & Ramshaw, I.A. IFN-γ plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 157, 3223–3227 (1996).
Krakowski, M. & Owens, T. Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 26, 1641–1646 (1996).
Willenborg, D.O., Fordham, S.A., Staykova, M.A., Ramshaw, I.A. & Cowden, W.B. IFN-γ is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J. Immunol. 163, 5278–5286 (1999).
Tran, E.H., Prince, E.N. & Owens, T. IFN-γ shapes immune invasion of the central nervous system via regulation of chemokines. J. Immunol. 164, 2759–2768 (2000).
Cua, D.J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Bettelli, E. & Kuchroo, V.K. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J. Exp. Med. 201, 169–171 (2005).
Harrington, L.E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Aggarwal, S. & Gurney, A.L. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 71, 1–8 (2002).
Moseley, T.A., Haudenschild, D.R., Rose, L. & Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 (2003).
Kolls, J.K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Ye, P. et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527 (2001).
Matusevicius, D. et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 5, 101–104 (1999).
Wong, C.K., Ho, C.Y., Li, E.K. & Lam, C.W. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 9, 589–593 (2000).
Hashimoto, T., Akiyama, K., Kobayashi, N. & Mori, A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int. Arch. Allergy Immunol. 137, 51–54 (2005).
Linden, A., Hoshino, H. & Laan, M. Airway neutrophils and interleukin-17. Eur. Respir. J. 15, 973–977 (2000).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177 (2003).
Bush, K.A., Farmer, K.M., Walker, J.S. & Kirkham, B.W. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 46, 802–805 (2002).
Komiyama, Y. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 (2006).
Hofstetter, H.H. et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 237, 123–130 (2005).
Liang, S.C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Wolk, K. & Sabat, R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 17, 367–380 (2006).
Chung, Y. et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 16, 902–907 (2006).
Zheng, Y. et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Aggarwal, S., Ghilardi, N., Xie, M.H., de Sauvage, F.J. & Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Langrish, C.L. et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 202, 96–105 (2004).
Parham, C. et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168, 5699–5708 (2002).
Mullen, A.C. et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292, 1907–1910 (2001).
Sutton, C., Brereton, C., Keogh, B., Mills, K.H. & Lavelle, E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691 (2006).
Liu, X.K., Clements, J.L. & Gaffen, S.L. Signaling through the murine T cell receptor induces IL-17 production in the absence of costimulation, IL-23 or dendritic cells. Mol. Cells 20, 339–347 (2005).
Nakae, S. et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA 100, 5986–5990 (2003).
Pflanz, S. et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16, 779–790 (2002).
Hunter, C.A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5, 521–531 (2005).
Batten, M. et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17–producing T cells. Nat. Immunol. 7, 929–936 (2006).
Stumhofer, J.S. et al. Interleukin 27 negatively regulates the development of interleukin 17–producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7, 937–945 (2006).
Chen, Y. et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116, 1317–1326 (2006).
Rangachari, M. et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 203, 2009–2019 (2006).
Mathur, A.N. et al. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood 108, 1595–1601 (2006).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Dzhagalov, I., Zhang, N. & He, Y.W. The roles of orphan nuclear receptors in the development and function of the immune system. Cell. Mol. Immunol. 1, 401–407 (2004).
Eberl, G. & Littman, D.R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Akimzhanov, A.M., Yang, X.O. & Dong, C. Chromatin remodeling at IL17-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. published online ahead of print 11 January 2007 (doi:10.1074/jbc.C600322200).
Powrie, F. & Coffman, R.L. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol. Today 14, 270–274 (1993).
Khoury, S.J., Hancock, W.W. & Weiner, H.L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J. Exp. Med. 176, 1355–1364 (1992).
Merrill, J.E. et al. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc. Natl. Acad. Sci. USA 89, 574–578 (1992).
Chitnis, T. et al. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J. Clin. Invest. 108, 739–747 (2001).
Bettelli, E. et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 200, 79–87 (2004).
Lovett-Racke, A.E. et al. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity 21, 719–731 (2004).
Panitch, H.S. Interferons in multiple sclerosis. A review of the evidence. Drugs 44, 946–962 (1992).
Jones, L.S. et al. IFN-γ-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J. Immunol. 158, 5997–6005 (1997).
Murphy, C.A. et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Segal, B.M., Dwyer, B.K. & Shevach, E.M. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med. 187, 537–546 (1998).
Kaplan, M.H., Sun, Y.L., Hoey, T. & Grusby, M.J. Impaired IL-12 responses and enhanced development of TH2 cells in Stat4-deficient mice. Nature 382, 174–177 (1996).
Szabo, S.J. et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 (2000).
Korn, T. et al. Myelin-specific regulatory T-cells accumulate in the central nervous system, but fail to suppress pathogenic effector T-cells at the peak of autoimmune inflammation. Nat. Med. (in the press).
Kim, J.M., Rasmussen, J.P. & Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Peng, Y., Laouar, Y., Li, M.O., Green, E.A. & Flavell, R.A. TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. USA 101, 4572–4577 (2004).
Kretschmer, K. et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6, 1219–1227 (2005).
Lohr, J., Knoechel, B., Wang, J.J., Villarino, A.V. & Abbas, A.K. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J. Exp. Med. 203, 2785–2791 (2006).
Woo, P. et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res. Ther. 7, R1281–R1288 (2005).
Maini, R.N. et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 54, 2817–2829 (2006).
Acknowledgements
Supported by the National Multiple Sclerosis Society (V.K.K.; RG3882-A-1 to M.O.; TA 3014A1/1 to E.B.), the National Institutes of Health and the Juvenile Diabetes Foundation (V.K.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bettelli, E., Oukka, M. & Kuchroo, V. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol 8, 345–350 (2007). https://doi.org/10.1038/ni0407-345
Issue Date:
DOI: https://doi.org/10.1038/ni0407-345
This article is cited by
-
Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics
Experimental & Molecular Medicine (2023)
-
Matrine exerts its neuroprotective effects by modulating multiple neuronal pathways
Metabolic Brain Disease (2023)
-
Maternal immune activation during pregnancy is associated with more difficulties in socio-adaptive behaviors in autism spectrum disorder
Scientific Reports (2023)
-
Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases
Cell Death & Disease (2022)
-
Histone deacetylase 6 plays an important role in TGF-β-induced murine Treg cell differentiation by regulating cell proliferation
Scientific Reports (2022)