Abstract
Siglec-9 is a sialic-acid-binding lectin expressed predominantly on myeloid cells. Aberrant glycosylation occurs in essentially all types of cancers and results in increased sialylation. Thus, when the mucin MUC1 is expressed on cancer cells, it is decorated by multiple short, sialylated O-linked glycans (MUC1-ST). Here we found that this cancer-specific MUC1 glycoform, through engagement of Siglec-9, ‘educated’ myeloid cells to release factors associated with determination of the tumor microenvironment and disease progression. Moreover, MUC1-ST induced macrophages to display a tumor-associated macrophage (TAM)-like phenotype, with increased expression of the checkpoint ligand PD-L1. Binding of MUC1-ST to Siglec-9 did not activate the phosphatases SHP-1 or SHP-2 but, unexpectedly, induced calcium flux that led to activation of the kinases MEK-ERK. This work defines a critical role for aberrantly glycosylated MUC1 and identifies an activating pathway that follows engagement of Siglec-9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Quail, D.F. & Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Kitamura, T., Qian, B.Z. & Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 (2015).
Pinho, S.S. & Reis, C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015).
Burchell, J.M., Mungul, A. & Taylor-Papadimitriou, J. O-linked glycosylation in the mammary gland: changes that occur during malignancy. J. Mammary Gland Biol. Neoplasia 6, 355–364 (2001).
Gendler, S.J. et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 265, 15286–15293 (1990).
Burchell, J. et al. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 9, 1307–1311 (1999).
Lloyd, K.O., Burchell, J., Kudryashov, V., Yin, B.W. & Taylor-Papadimitriou, J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J. Biol. Chem. 271, 33325–33334 (1996).
Beatson, R. et al. The breast cancer-associated glycorms of MUC1, MUC1-Tn and sialyl-Tn, are expressed in COSMC wild-type cells and bind the C-type lectin MGL. PLoS One 10, e0125994 (2015).
Mungul, A. et al. Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int. J. Oncol. 25, 937–943 (2004).
Picco, G. et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 20, 1241–1250 (2010).
Macauley, M.S., Crocker, P.R. & Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 (2014).
Avril, T., Floyd, H., Lopez, F., Vivier, E. & Crocker, P.R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 173, 6841–6849 (2004).
Crocker, P.R., Paulson, J.C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 (2007).
Jandus, C. et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Invest. 124, 1810–1820 (2014).
Läubli, H. et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl. Acad. Sci. USA 111, 14211–14216 (2014).
Hudak, J.E., Canham, S.M. & Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 10, 69–75 (2014).
Tanida, S. et al. Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth. J. Biol. Chem. 288, 31842–31852 (2013).
Bäckström, M. et al. Recombinant MUC1 mucin with a breast cancer-like O-glycosylation produced in large amounts in Chinese-hamster ovary cells. Biochem. J. 376, 677–686 (2003).
Zhang, J.Q., Nicoll, G., Jones, C. & Crocker, P.R. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J. Biol. Chem. 275, 22121–22126 (2000).
Carlin, A.F. et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113, 3333–3336 (2009).
Dalziel, M. et al. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem. 276, 11007–11015 (2001).
Qian, B.Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Thapa, B., Koo, B.H., Kim, Y.H., Kwon, H.J. & Kim, D.S. Plasminogen activator inhibitor-1 regulates infiltration of macrophages into melanoma via phosphorylation of FAK-Tyr925. Biochem. Biophys. Res. Commun. 450, 1696–1701 (2014).
McMahon, G.A. et al. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 276, 33964–33968 (2001).
Bauerle, K.T. et al. Nuclear factor κB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8. J. Clin. Endocrinol. Metab. 99, E1436–E1444 (2014).
Chen, H. et al. Silencing of plasminogen activator inhibitor-1 suppresses colorectal cancer progression and liver metastasis. Surgery 158, 1704–1713 (2015).
Thompson, P.A. et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis 36 (Suppl. 1), S232–S253 (2015).
Oosterhoff, D. et al. Tumor-mediated inhibition of human dendritic cell differentiation and function is consistently counteracted by combined p38 MAPK and STAT3 inhibition. OncoImmunology 1, 649–658 (2012).
Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 36, 161–178 (2015).
Murray, P.J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Rughetti, A. et al. Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation and function of dendritic cells. J. Immunol. 174, 7764–7772 (2005).
Allavena, P. & Mantovani, A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin. Exp. Immunol. 167, 195–205 (2012).
Forouzandeh, F., Jalili, R.B., Germain, M., Duronio, V. & Ghahary, A. Differential immunosuppressive effect of indoleamine 2,3-dioxygenase (IDO) on primary human CD4+ and CD8+ T cells. Mol. Cell. Biochem. 309, 1–7 (2008).
Gianchecchi, E., Delfino, D.V. & Fierabracci, A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun. Rev. 12, 1091–1100 (2013).
Sousa, S. et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 17, 101 (2015).
Qian, B.Z. & Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Paul, S.P., Taylor, L.S., Stansbury, E.K. & McVicar, D.W. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 96, 483–490 (2000).
Christo, S.N., Diener, K.R. & Hayball, J.D. The functional contribution of calcium ion flux heterogeneity in T cells. Immunol. Cell Biol. 93, 694–704 (2015).
Dudley, D.T., Pang, L., Decker, S.J., Bridges, A.J. & Saltiel, A.R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92, 7686–7689 (1995).
Xuan, Q.J. et al. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol. Oncol. Res. 20, 619–624 (2014).
Noy, R. & Pollard, J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Garon, E.B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Casey, S.C. et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 352, 227–231 (2016).
Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Belisle, J.A. et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol. Cancer 9, 118 (2010).
Ohta, M. et al. Immunomodulation of monocyte-derived dendritic cells through ligation of tumor-produced mucins to Siglec-9. Biochem. Biophys. Res. Commun. 402, 663–669 (2010).
Correa, I. et al. Responses of human T cells to peptides flanking the tandem repeat and overlapping the signal sequence of MUC1. Int. J. Cancer 115, 760–768 (2005).
Priatel, J.J. et al. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity 12, 273–283 (2000).
Julien, S. et al. Sialyl-Lewis(x) on P-selectin glycoprotein ligand-1 is regulated during differentiation and maturation of dendritic cells: a mechanism involving the glycosyltransferases C2GnT1 and ST3Gal I. J. Immunol. 179, 5701–5710 (2007).
Sproviero, D., Julien, S., Burford, B., Taylor-Papadimitriou, J. & Burchell, J.M. Cyclooxygenase-2 enzyme induces the expression of the α-2,3-sialyltransferase-3 (ST3Gal-I) in breast cancer. J. Biol. Chem. 287, 44490–44497 (2012).
Acknowledgements
We thank V. Corrigall (King's College London) for tocilizumab; and N. O'Reilly for the lyophilization of samples. Supported by Breast Cancer Now (2011NovPR-43), the Medical Research Council (MR/J007196/1), the Department the Experimental Cancer Medicine Centre at King's College London, the National Institute for Health Research Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Author information
Authors and Affiliations
Contributions
R.B. and J.M.B. designed the study and wrote the manuscript with comments from all authors; R.B. performed the experiments with the assistance of D.A., G.P., T.-D.T. and M.H.; V.T.-O. performed the quantitative RT-PCR; S.K. and T.N. cultured the Chinese hamster ovary cells in bulk; J.M. and P.R.C. supplied reagents; and J.T.-P. contributed to scientific discussions and approaches.
Corresponding author
Ethics declarations
Competing interests
J.M.B. is a consultant to Palleon Pharma, and P.R.C. is a scientific co-founder of Palleon Pharma.
Integrated supplementary information
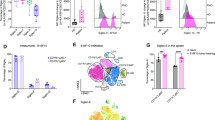
Supplementary Figure 1 MUC1-ST binds to Siglec-9.
(a) Representative flow cytometry histograms showing biotinylated MUC1-T or MUC1-ST binding to isolated or differentiated immune subsets from healthy donors. N=4 independent donors. (b,c) Monocytes were incubated with biotinylated MUC1-ST for (b) different time periods or (c) using different concentrations. N=2 independent donors. (d) Monocytes were treated ± neuraminidase before being incubated with 10µg/ml MUC1 glycoforms. N=3 independent donors. (e) Monocytes (N=3) and MCSF MΦ (N=2) were stained with antibodies to MUC1-ST binding Siglecs and analysed by flow cytometry. (f) Monocytes were treated with indicated concentrations of antibodies to Siglecs-3, 7 and 9 before being incubated with MUC1-ST. Graph illustrates % binding inhibition as calculated against MUC1-ST plus isotype MFI. N=3 independent donors. (g) Representative flow cytometry histograms showing the binding of MUC1-ST or PAA-ST in the presence of anti-Siglec-9 or isotype for both primary monocytes and U937 cells. Data shown are the mean and s.e.m. * p<0.05 ** p<0.01 *** p<0.001, paired or unpaired Student’s t-test where appropriate.
Supplementary Figure 2 MUC1-ST induces monocytes to secrete factors associated with tumor progression and modulates the differentiation of monocytes into dendritic cells (moDCs).
(a) The supernatant from MUC1-ST educated monocytes was analysed using a protein array. Highlighted factors: 1, CXCL5, 2, Chitinase 3-like 1, 3 IL-8, 4 CCL3, 5 IL17A, 6 MMP-9, 7 CCL2, 8 PAI-1, 9 IL6, 10 CXCL1. (b,c) Pooled MFI data showing CD40, CD83, HLA-DR and CD86 expression on day 7 immature (b) or mature (c) moDCs after treatment with MUC1-ST on day 0. N=6 independent donors. (d) Representative flow cytometry histograms showing CD86 expression on day 7 immature or mature moDCs after treatment with MUC1-ST on day 0 in the presence of isotype or anti Siglec-9 or anti IL-6Rα. (e,f,g) Pooled cytometric data showing CD86 (e,f) and CD83 (g) expression on day 7 immature (e) or mature (f,g) moDCs after treatment with MUC1-ST on day 0 in the presence of isotype or anti-Siglec-9 or anti-IL-6Rα. N=6 independent donors. (h) IL-12 p70 release from day 7 mature moDCs after treatment with MUC1-ST on day 0 in the presence of isotype or anti-Siglec-9 or anti-IL-6Rα. N= 6 independent donors. Data shown are the mean and s.e.m. * p<0.05 ** p<0.01 *** p<0.001, paired or unpaired Student’s t-test where appropriate.
Supplementary Figure 3 Proposed model of MUC1-ST-mediated modulation of the tumor microenvironment via engagement of Siglec-9.
Transformation associated inflammation induces the expression of MUC1 and COX-2 which in turn upregulates ST3Gal-I49. This increases the presence of MUC1-ST, which via Siglec-9, educates monocytes and macrophages to release factors involved in immune recruitment and tumor progression. Additionally, Siglec-9 engagement by MUC1-ST on monocytes results in altered differentiation and a dysfunctional phenotype, and on macrophages induces a TAM-like phenotype with increased expression of CD206, CD163, IDO and PD-L1, and poor CD8+ co-stimulatory ability. This cycle is maintained by MUC1-ST/Siglec-9 induced factors being able to upregulate ST3Gal-1 and MUC1, thus ensuring conservation of the microenvironment.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Tables 1 and 2 (PDF 976 kb)
Rights and permissions
About this article
Cite this article
Beatson, R., Tajadura-Ortega, V., Achkova, D. et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol 17, 1273–1281 (2016). https://doi.org/10.1038/ni.3552
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3552
This article is cited by
-
A mucin degrader for cancer therapy
Nature Biotechnology (2024)
-
Design of a mucin-selective protease for targeted degradation of cancer-associated mucins
Nature Biotechnology (2024)
-
Polymeric biomaterial-inspired cell surface modulation for the development of novel anticancer therapeutics
Biomaterials Research (2023)
-
Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment
Molecular Cancer (2023)
-
Exploiting innate immunity for cancer immunotherapy
Molecular Cancer (2023)