Abstract
Mitochondria need to be juxtaposed to phagosomes for the synergistic production of ample reactive oxygen species (ROS) in phagocytes to kill pathogens. However, how phagosomes transmit signals to recruit mitochondria has remained unclear. Here we found that the kinases Mst1 and Mst2 functioned to control ROS production by regulating mitochondrial trafficking and mitochondrion-phagosome juxtaposition. Mst1 and Mst2 activated the GTPase Rac to promote Toll-like receptor (TLR)-triggered assembly of the TRAF6-ECSIT complex that is required for the recruitment of mitochondria to phagosomes. Inactive forms of Rac, including the human Rac2D57N mutant, disrupted the TRAF6-ECSIT complex by sequestering TRAF6 and substantially diminished ROS production and enhanced susceptibility to bacterial infection. Our findings demonstrate that the TLR-Mst1-Mst2-Rac signaling axis is critical for effective phagosome-mitochondrion function and bactericidal activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
West, A.P., Koblansky, A.A. & Ghosh, S. Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 22, 409–437 (2006).
Beutler, B. et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24, 353–389 (2006).
Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 (2004).
Bedard, K. & Krause, K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Bokoch, G.M. & Diebold, B.A. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100, 2692–2696 (2002).
Mehta, D., Rahman, A. & Malik, A.B. Protein kinase C-α signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 276, 22614–22620 (2001).
DerMardirossian, C., Schnelzer, A. & Bokoch, G.M. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol. Cell 15, 117–127 (2004).
Ambruso, D.R. et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl. Acad. Sci. USA 97, 4654–4659 (2000).
Williams, D.A. et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood 96, 1646–1654 (2000).
Gu, Y. et al. Biochemical and biological characterization of a human Rac2 GTPase mutant associated with phagocytic immunodeficiency. J. Biol. Chem. 276, 15929–15938 (2001).
West, A.P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Sena, L.A. & Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Emre, Y. et al. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem. J. 402, 271–278 (2007).
Wu, W., Hsu, Y.M., Bi, L., Songyang, Z. & Lin, X. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat. Immunol. 10, 1208–1214 (2009).
Arsenijevic, D. et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 26, 435–439 (2000).
Bulua, A.C. et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533 (2011).
Creasy, C.L., Ambrose, D.M. & Chernoff, J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J. Biol. Chem. 271, 21049–21053 (1996).
Creasy, C.L. & Chernoff, J. Cloning and characterization of a human protein kinase with homology to Ste20. J. Biol. Chem. 270, 21695–21700 (1995).
Rawat, S.J. & Chernoff, J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem. Sci. 40, 149–156 (2015).
Avruch, J. et al. Protein kinases of the Hippo pathway: regulation and substrates. Semin. Cell Dev. Biol. 23, 770–784 (2012).
Zhou, D. et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009).
O'Neill, E., Rushworth, L., Baccarini, M. & Kolch, W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 306, 2267–2270 (2004).
Del Re, D.P. et al. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol. Cell 54, 639–650 (2014).
Maejima, Y. et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat. Med. 19, 1478–1488 (2013).
Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Harvey, K.F., Zhang, X. & Thomas, D.M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Yu, F.X. & Guan, K.L. The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 (2013).
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 (2014).
Zeng, Q. & Hong, W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13, 188–192 (2008).
Zhou, D. et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. USA 108, E1312–E1320 (2011).
Abdollahpour, H. et al. The phenotype of human STK4 deficiency. Blood 119, 3450–3457 (2012).
Nehme, N.T. et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 119, 3458–3468 (2012).
Du, X. et al. Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1/Foxo3 stability in autoimmune disease. J. Immunol. 192, 1525–1535 (2014).
Dong, Y. et al. A cell-intrinsic role for Mst1 in regulating thymocyte egress. J. Immunol. 183, 3865–3872 (2009).
Ueda, Y. et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat. Commun. 3, 1098 (2012).
Katagiri, K., Imamura, M. & Kinashi, T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat. Immunol. 7, 919–928 (2006).
Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4, 741–748 (2003).
Katagiri, K. et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat. Immunol. 5, 1045–1051 (2004).
Zhou, D. et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naive T cells. Proc. Natl. Acad. Sci. USA 105, 20321–20326 (2008).
Mou, F. et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J. Exp. Med. 209, 741–759 (2012).
Raab, M. et al. T cell receptor “inside-out” pathway via signaling module SKAP1-RapL regulates T cell motility and interactions in lymph nodes. Immunity 32, 541–556 (2010).
Sasai, M. & Yamamoto, M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int. Rev. Immunol. 32, 116–133 (2013).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Jiang, X. & Chen, Z.J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 12, 35–48 (2012).
Jiao, S. et al. The kinase MST4 limits inflammatory responses through direct phosphorylation of the adaptor TRAF6. Nat. Immunol. 16, 246–257 (2015).
Lehtinen, M.K. et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125, 987–1001 (2006).
Yuan, Z. et al. Regulation of neuronal cell death by MST1-FOXO1 signaling. J. Biol. Chem. 284, 11285–11292 (2009).
Xiao, L. et al. The c-Abl-MST1 signaling pathway mediates oxidative stress-induced neuronal cell death. J. Neurosci. 31, 9611–9619 (2011).
Praskova, M., Xia, F. & Avruch, J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 18, 311–321 (2008).
Torrino, S. et al. The E3 ubiquitin-ligase HACE1 catalyzes the ubiquitylation of active Rac1. Dev. Cell 21, 959–965 (2011).
Acknowledgements
Supported by the National Basic Research Program (973) of China (2015CB910502 to L.C.), China's 1000 Young Talents Program (D.Z. and L.C.), the 111 Projects (B12001 and B06016), the Fundamental Research Funds for the Central Universities of China-Xiamen University (CXB2014004 to J.Z.; 20720140551 to L.C.; and 2013121034 and 20720140537 to D.Z.), the National Natural Science Foundation of China (31270918, 81222030 and J1310027 to D.Z.; 81372617, 81422018 and U1405225 to L.C.; 81472229 to L.H.; and 81302529 to X.L.), the Natural Science Foundation of Fujian (2013J06011 to D.Z. and 2014D007 to X.L.), the US National Institutes of Health (RO1 CA136567 for J.A.) and institutional funds from Massachusetts General Hospital (for J.A.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.G., X.S., L.C. and D.Z. designed the research and helped with data analysis; J.G., X.S., P.W., S.Z., X.W., H.W., L.H., C.X., X.L., H.Z., Q.L., M.J., Q.C., J.Z., Y.L. and K.-Y.C. performed the experiments and helped with data analysis; S.S., H.-R.W., R.Z., R.L.J., S.-C.L., J.H. and J.A. contributed to discussions and provided critical reagents; and J.A., L.C. and D.Z. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
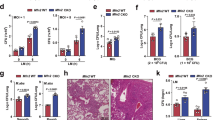
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis.
(a) H&E staining of colon and kidney sections from wild type and Mst1-/-Mst2fl/flVav-Cre mice. Scale bar, 50 μm.
(b, c) Representative spleen tissues: spleen weight (b) and H&E staining of spleen sections (c) from wild type and Mst1-/-Mst2fl/fl Vav-Cre mice. Scale bar, 100 μm.
(d) White blood cell (WBC), circulating lymphocyte (LYM), monocyte (MON) and granulocyte (GRA) counts in Mst1fl/flMst2fl/fl (WT) and Mst1fl/flMst2fl/flLyz2-Cre (cDKO) mice. Each dot represents an individual mouse, and horizontal bars indicate the mean.
(e) Flow cytometric analysis of Gr-1+CD11b+ neutrophil and F4/80+CD11b+ macrophage populations in the bone marrow and spleens of WT and cDKO mice, as determined with anti-Gr-1, anti-F4/80 and anti-CD11b antibodies.
(f) Flow cytometric analysis of B220+ B cell, CD3+ T cell, the naïve T cell (CD62LhighCD44low) and the effector T cell (CD62LlowCD44high) populations in the spleen, lymph node (LN) or blood of WT and cDKO mice, with the indicated antibodies.
(g) Flow cytometric analysis of Gr-1+CD11b+ neutrophil and F4/80+CD11b+ macrophage populations in blood of WT and cDKO mice subjected to cecal ligation and puncture (CLP) for 24h.
(h) H&E staining of kidney sections from WT or cDKO mice subjected to sublethal CLP. Scale bar, 50 μm.
(i) WT BMDMs were stimulated with different TLR agonists for the indicated times, followed by immunoblot analysis with the indicated antibodies.
Data were assessed with Student’s t-test and are represented as mean ± s.d. ns, not significant, * p<0.05, *** p<0.001 compared with respective controls or as indicated (b, n=10; d, n=8; e, n=3; g, n=4). Data are representative of three independent experiments.
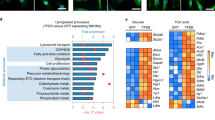
Supplementary Figure 2 Mst1- and Mst2-deficient myeloid cells are defective in killing bacteria and induction of mROS.
(a) Mst1fl/flMst2fl/fl (WT) and Mst1fl/flMst2fl/flLyz2-Cre (cDKO) BMDMs or neutrophils were treated with indicated TLR agonists for 6 h. LPS, Lipopolysaccharide; Pam3, Pam3CSK4; LTA, Lipoteichoic acid; PIC, poly(I:C). mROS (MitoSox) and total cellular ROS (CM-H2DCFDA) production were measured by flow cytometry.
(b) Luminometry of ROS production by WT and cDKO BMDMs or neutrophils infected with E. coli (MOI, 10) or L. monocytogenes (Lm, MOI, 10). RU, relative units. Results are represented as mean ± s.d. n=3.
(c) WT and cDKO BMDMs or neutrophils were treated with antimycin A, oligomysin or rotenone for 6 h. mROS production was measured as described in (a).
(d-f) Representative transmission electron micrographs (d, Scale bar, 0.5 μm), the number of mitochondria (d), western blots of the major electron transport chain components (e) and the mitochondrial potential shown by JC-1 staining (f, Scale bar, 50 μm) of DMDMs from WT and cDKO mice. Data were assessed with Student’s t test and are represented as mean ± s.d. (d, n=20). ns, not significant.
Data are representative of three independent experiments.
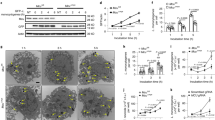
Supplementary Figure 3 Treatment with cytochalasin D diminishes the induction of mROS and cellular ROS in BMDMs after stimulation with LPS.
(a) Mst1fl/flMst2fl/fl (WT), Mst1fl/flMst2fl/flLyz2-Cre (cDKO), Rac1G12V and cDKO-Rac1G12V BMDMs were treated with the indicated doses of cytochalasin D and were immunostained with F-actin antibodies (green). Scale bar, 20 μm.
(b) WT BMDMs were pretreated with the indicated doses of cytochalasin D for 1 h followed by LPS stimulation for 3 h. mROS and cellular ROS production were measured by staining cells with MitoSOX and CM-H2DCFDA for 30 min, respectively, followed by flow cytometry.
Data are representative of three independent experiments.
Supplementary Figure 4 Interaction of Mst2 and PKC isoforms.
(a) The interaction between Mst2 and PKC kinases. Co-immunoprecipitation assay of 293T cells expressing HA-Mst2 and Flag-tagged PKCa, b, g, d, e, h, q, i or z as indicated. Cell lysates were immunoprecipitated with anti-Flag antibody and analysed by immunoblotting with antibodies as indicated.
(b) Mst2 binds the N-terminal regulatory domain of PKCα. Co-immunoprecipitation assay of 293T cells expressing HA-Mst2 and Flag-PKCα N-terminal (NT) or Flag-PKCα C-terminal (CT) as indicated combination. The cell lysates and immunoprecipitates were analysed by immunoblotting with antibodies as indicated.
(c) Phosphorylation sites of PKCα were detected by mass spectrometry analysis of bacterially expressed GST-tagged PKCα(NT) from an in vitro kinase reaction with triple HA-tagged Mst2 immunoprecipitated from HeLa cells.
Data are representative of three independent experiments.
Supplementary Figure 5 TRAF6 activity is essential for the K63-linked ubiquitylation of Rac1.
(a) The distribution of Rac1 (red) in Mst1fl/flMst2fl/fl (WT) BMDMs, Mst1fl/flMst2fl/flLyz2-Cre (cDKO) BMDMs and WT BMDMs treated with NSC23766 after phagocytosis with GFP-E. coli. Images shown are representative of approximately 100 cells. Scale bar, 20 μm.
(b) Pull-down experiments were performed using purified Flag-Rac1WT, Flag-Rac1G12V or Flag-RacT17N incubated with lysates from LPS-treated BMDMs. Immunoblots show the association of TRAF6 with Flag-RacT17N.
(c) Ubiquitination and immunoprecipitation assay of 293T cells transfected with plasmids expressing Flag-Rac1, Myc-TRAF6 and HA-ubiquitin (Ub), HA-ubiquitin K48 (Ub 48) or HA-ubiquitin K63 (Ub 63). Cell lysates were immunoprecipitated with anti-Flag antibody. The cell lysates and immunoprecipitates were analysed by immunoblotting with antibodies as indicated. Anti-HA immunoblotting shows the level of Rac ubiquitylation.
(d) Knock-down of TRAF6 expression levels in BMDMs or RAW264.7 cells with different TRAF6 shRNAs.
(e) The ubiquitination levels of WT or mutated Rac1 with the indicated substitutions of lysine (K) with arginine (R) in 293T cells.
Data are representative of three independent experiments.
Supplementary Figure 6 TRAF6 maintains a Rac1 activation state via K63-linked ubiquitination.
(a) HeLa cells were transfected with plasmids expressing Rac1WT, Rac1G12V or Rac1TN17, and/or TRAF6 as indicated combination. Confocal microscopy shows the co-localization of TRAF6 (green) with Rac1TN17 (red), but not with Rac1G12V (red), at the cell periphery. Images shown are representative of approximately 100 cells. Scale bar, 20 μm.
(b) Ubiquitination and immunoprecipitation assay of 293T cells expressing Flag-Rac1, Myc-TRAF6 and/or HA-Ub and treated with or without NSC23766 as indicated. Cell lysates were immunoprecipitated with anti-Flag antibody and analysed by immunoblotting with antibodies as indicated. Anti-HA immunoblotting shows that Rac ubiquitination by TRAF6 is reduced by the Rac1 inhibitor.
Data are representative of three independent experiments.
Supplementary Figure 7 Rac1G12V enhances assembly of the TRAF6-ECSIT complex.
(a-c) ECIST or Rac1 binds to the math domain of TRAF6. Co-immunoprecipitation assay of 293T cells expressing HA-ECSIT (a) or HA-Rac1T17N (b), and Flag-TRAF6 full-length (FL) or fragments as indicated. Cell lysates were immunoprecipitated with anti-Flag antibody and analysed by immunoblotting with antibodies as indicated. Schematic representation of the domain organization of TRAF6 is shown (c).
(d) Loss of the K63-linked ubiquitination of inactivated Rac2D57N by TRAF6 in 293T cells. Ubiquitination and co-immunoprecipitation assay of 293T cells expressing Flag-Rac2WT, Flag-Rac2D57N and/or Myc-TRAF6, plus WT ubiquitin (HA-Ub) or K63-linked ubiquitin ([63 only]HA-Ub) as indicated.
(e) HeLa cells were transfected with expression plasmids for Rac1WT, Rac1G12V or Rac1TN17. Confocal microscopy shows enhanced co-localization of TRAF6 (green) with ECSIT (red) mediated by active Rac1G12V (purple). Images shown are representative of approximately 100 cells. Scale bar, 20 μm.
Data are representative of three independent experiments.
Supplementary Figure 8 Mst1 and Mst2 positively regulate the induction of phagocyte ROS and bactericidal activity.
(a) A proposed working model for TLR-Hippo signalling-mediated ROS production. During an antimicrobial response, Toll-like receptor (TLR) signalling triggers the Mst1 and Mst2 kinases to directly phosphorylate PKCa at Ser 226 and Thr 228, leading to its activation, which promotes the dissociation of the GDP-dissociation inhibitor LyGDI from Rac and enhances the GTP charging of Rac. Activated Rac then triggers the assembly of the TRAF6-ECSIT complex, controlling mitochondrial trafficking and promoting mitochondrion-phagosome juxtaposition and thereby augmenting mitochondrial ROS (mROS) production. TRAF6 further mediates the Lys (63)-linked ubiquitination of active Rac, maintaining the GTP charging of Rac and the subsequent activation of ROS-generating machinery during an antimicrobial response.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 815 kb)
Supplementary Table
Supplementary Table 1 (PDF 180 kb)
Rights and permissions
About this article
Cite this article
Geng, J., Sun, X., Wang, P. et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol 16, 1142–1152 (2015). https://doi.org/10.1038/ni.3268
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3268
This article is cited by
-
Innate immune and proinflammatory signals activate the Hippo pathway via a Tak1-STRIPAK-Tao axis
Nature Communications (2024)
-
The role of non-canonical Hippo pathway in regulating immune homeostasis
European Journal of Medical Research (2023)
-
A diagnostic model for sepsis-induced acute lung injury using a consensus machine learning approach and its therapeutic implications
Journal of Translational Medicine (2023)
-
Trained immunity — basic concepts and contributions to immunopathology
Nature Reviews Nephrology (2023)
-
Near-infrared light reduces β-amyloid-stimulated microglial toxicity and enhances survival of neurons: mechanisms of light therapy for Alzheimer’s disease
Alzheimer's Research & Therapy (2022)