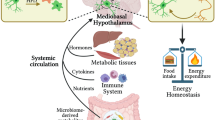
Abstract
Innate immunological signals induced by pathogen- and/or damage-associated molecular patterns are essential for adaptive immune responses, but it is unclear if the brain has a role in this process. Here we found that while the abundance of tumor-necrosis factor (TNF) quickly increased in the brain of mice following bacterial infection, intra-brain delivery of TNF mimicked bacterial infection to rapidly increase the number of peripheral lymphocytes, especially in the spleen and fat. Studies of various mouse models revealed that hypothalamic responses to TNF were accountable for this increase in peripheral lymphocytes in response to bacterial infection. Finally, we found that hypothalamic induction of lipolysis mediated the brain's action in promoting this increase in the peripheral adaptive immune response. Thus, the brain-fat axis is important for rapid linkage of innate immunity to adaptive immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Beutler, B. Innate immunity: an overview. Mol. Immunol. 40, 845–859 (2004).
Janeway, C.A. Jr. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Rubartelli, A. & Lotze, M.T. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 28, 429–436 (2007).
Hoebe, K., Janssen, E. & Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 5, 971–974 (2004).
Kabelitz, D. & Medzhitov, R. Innate immunity–cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr. Opin. Immunol. 19, 1–3 (2007).
Banyer, J.L., Hamilton, N.H., Ramshaw, I.A. & Ramsay, A.J. Cytokine in innate and adaptive immunity. Rev. Immunogenet. 2, 359–373 (2000).
Purkayastha, S. et al. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc. Natl. Acad. Sci. USA 108, 2939–2944 (2011).
Purkayastha, S., Zhang, G. & Cai, D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 17, 883–887 (2011).
Zhang, G. et al. Hypothalamic programing of system ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Li, J., Tang, Y. & Cai, D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 14, 999–1012 (2012).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Hamon, M., Bierne, H. & Cossart, P. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4, 423–434 (2006).
Pamer, E.G. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4, 812–823 (2004).
Gross, P.M. Circumventricular organ capillaries. Prog. Brain Res. 91, 219–233 (1992).
McCusker, R.H. & Kelly, K.W. Immune-neural connections: how the immune system's response to infectious agents influences behavior. J. Exp. Biol. 216, 84–98 (2013).
Meng, Q. & Cai, D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IκB kinase beta (IKKβ)/NF-κB pathway. J. Biol. Chem. 286, 32324–32332 (2011).
Peschon, J.J. et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160, 943–952 (1998).
Chen, C.P., Hertzberg, M., Jiang, Y. & Graves, D.T. Interleukin-1 and tumor necrosis factor receptor signaling is not required for bacteria-induced osteoclastogenesis and bone loss but is essential for protecting the host from a mixed anaerobic infection. Am. J. Pathol. 155, 2145–2152 (1999).
Nguyen, M.T. et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282, 35279–35292 (2007).
Saberi, M. et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Könner, A.C. & Bruning, J.C. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol. Metab. 22, 16–23 (2011).
Fessler, M.B., Rudel, L.L. & Brown, J.M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 20, 379–385 (2009).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Zu, L. et al. Bacterial endotoxin stimulates adipose lipolysis via toll-like receptor 4 and extracellular signal-regulated kinase pathway. J. Biol. Chem. 284, 5915–5926 (2009).
Youngstrom, T.G. & Bartness, T.J. White adipose tissue sympathetic nervous system denervation increase fat pad cell number. Am. J. Physiol. 275, R1488–R1493 (1998).
Cousin, B. et al. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology 133, 2255–2262 (1993).
Buettner, C. et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 14, 667–675 (2008).
Trotter-Mayo, R.N. & Roberts, M.R. Leptin acts in the periphery to protect thymocytes from glucocorticoid-mediated apoptosis in the absence of weight loss. Endocrinology 149, 5209–5218 (2008).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Wu, H. et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115, 1029–1038 (2007).
Yang, G., Parkhurst, C.N., Hayes, S. & Gan, W.B. Peripheral elevation of TNF-α leads to early synaptic abnormalities in the mouse somatosensory cortex in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 110, 10306–10311 (2013).
Meisel, C. et al. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 6, 775–786 (2005).
Huston, J.M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628 (2006).
Sun, J., Singh, V., Kajino-Sakamoto, R. & Aballay, A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 332, 729–732 (2011).
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiiflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 105, 11008–11013 (2008).
Pavlov, V.A. et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. USA 103, 5219–5223 (2006).
Posey, K.A. et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 296, E1003–E1012 (2009).
Thaler, J.P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Matarese, G. et al. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell response. Proc. Natl. Acad. Sci. USA 110, 6193–6198 (2013).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Winner, B., Kohl, Z. & Gage, F.H. Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 33, 1139–1151 (2011).
Berg, A.H. & Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 96, 939–949 (2005).
Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Sennello, J.A. et al. Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology 146, 2157–2164 (2005).
Stentz, F.B. & Kitabchi, A.E. Palmitic acid-induced activation of human T-lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. Biochem. Biophys. Res. Commun. 346, 721–726 (2006).
Xu, A.W. et al. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 115, 951–958 (2005).
Yan, J. et al. Obesity- and aging-induced excess of central transforming growth factor-beta potentiates diabetic development via an RNA stress response. Nat. Med. 20, 1001–1008 (2014).
Brunengraber, D.Z. et al. Influence of diet on the modeling of adipose tissue triglycerides during growth. Am. J. Physiol. Endocrinol. Metab. 285, E917–E925 (2003).
Acknowledgements
We thank the members of the laboratory of D.C. for technical assistance. Supported by the US National Institutes of Health (R01 DK078750, R01 AG031774, R01 HL113180 and R01 DK 099136 to D.C.).
Author information
Authors and Affiliations
Contributions
M.S.K. co-designed and performed all experiments, prepared all figures and provided writing assistance; J.Y. did viral injection, histology and immunostaining; W.W. contributed to animal generation, sample preparation and flow cytometry; G.Z. co-designed and did preliminary experiments for Figures 1 and 7; Y.Z. did viral cloning and generated viruses; M.S.K. and D.C. performed data analysis; D.C. conceived of the hypothesis and designed the project and structure of experiments and wrote the paper; and all authors participated in discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 CSF TNF concentrations and hypothalamic TNFR1 distribution.
(a) C57BL/6 mice were intravenously infected with Listeria monocytogenes (LM, +) vs. vehicle (–), and CSF samples were collected at Day 1 vs. 3 post infection for measuring TNF concentrations. (b) TNFR1 immunostaining (green) in the hypothalamus. The arcuate nucleus (ARC) and the medial ventral nucleus (VMH) were delineated. DAPI staining (blue) was used to reveal all cells in tissue sections. 3V: third ventricle. Images represent 3–4 mice per group. Scale bar = 500 μm. (c) Standard C57BL/6 mice were injected via pre-implanted third-ventricle cannula with a single dose (10 pg) of TNF, and CSF samples were collected at indicated time post injection for measuring TNF concentrations. ***P < 0.001 (ANOVA and Tukey’s post-hoc test); n = 5–6 mice per group. All results (mean) reflect two or three independent experiments with similar results (error bars, s.e.m.).
Supplementary Figure 2 Quantification of cell types of the immune system in mice after central injection of TNF or in mice during early bacterial infection.
C57BL/6 mice received daily injections of TNF (10 pg) (+) vs. vehicle (–) in the hypothalamic third-ventricle for 3 days, and then tissues were harvested for flow cytometry. Data show the numbers of F4/80+CD11c+ per gram of epididymal fat (a), spleen (b) and blood (c). Standard C57BL/6 mice were intravenously injected with Listeria monocytogenes (LM, +) vs. vehicle (–), and tissues were harvested at Day 1 or 3 for flow cytometry. Data show numbers of T cells (CD3+) (d,h), CD4+ T cells (CD3+CD4+) (e,i), CD8+ T cells (CD3+CD8+) (f,j) and B cells (B220+) (g,k) per gram of spleen (d–g) and epididymal fat (h–k). **P < 0.01, ***P < 0.001 (ANOVA and Tukey’s post-hoc test); n = 6–8 mice per group. All results (mean) reflect at least three independent experiments with similar results (error bars, s.e.m.).
Supplementary Figure 3 Effects of central injection of TNF on circulating lymphocytes or tissue T cell subtypes.
C57BL/6 mice received daily injections of TNF (10 pg) (+) vs. vehicle (–) in the hypothalamic third-ventricle for 3 days, and tissues were then harvested for flow cytometry. (a–d) Numbers of T cells (CD3+) (a), CD4+ T cells (CD3+CD4+) (b), CD8+ T cells (CD3+CD8+) (c) and B cells (B220+) (d) per gram of blood (n = 6–8 mice per group). (e–n) Numbers of ICOS+PD-1+ (e,g) and PD-L1+ (f,h) per gram of spleen (e,f) and epididymal fat (g,h), and numbers of CXCR3+CD4+CD3+ (i,l), CXCR3-CD4+CD3+ (j,m) and CCR6+CD4+CD3+ (k,n) per gram of spleen (i–k) and epididymal fat (l–n) (n = 4–6 mice per group). *P < 0.05, **P < 0.01 (two-tailed Student’s t-test); All results (mean) reflect two to three independent experiments with similar results (error bars, s.e.m.).
Supplementary Figure 4 Adaptive immune response or function by TNFRs in the hypothalamus and comprised Pomc neurons.
(a–d) Pomc-Cre mice received bilateral MBH injections with Cre-dependent lentiviral TNFR1 shRNA and TNFR2 shRNA (these mice are labeled as Pomc). Wild-type mice received bilateral MBH injections of lentiviral control were used for comparisons (these mice are labeled as WT). All these mice were injected with 10 pg TNF (+) via hypothalamic third-ventricle for 3 days. WT mice receiving vehicle injection in the hypothalamic third ventricle without viral injection (–) were used to provide the basal reference. Tissues were collected for flow cytometry. Data show numbers of T cells (CD3+) (a), CD4+ T cells (CD3+CD4+) (b), CD8+ T cells (CD3+CD8+) (c) and B cells (B220+) (d) per gram of epididymal fat (n = 3–5 mice per group). (e–g) TNFR-null (Tnfr-/-) mice vs. WT containing lentiviral TNFR1 (LV-T) or lentiviral control (LV-C) in the MBH received an intravenous injection of Listeria monocytogenes (LM, +) as described in Fig. 4, and were sacrificed at 3 days post LM injection for counting colony forming unit (CFU) in the spleen (e), epididymal fat (f) and liver (g) (n = 4–7 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA and Tukey’s post-hoc test); All results (mean) reflect two to three independent experiments with similar results (error bars, s.e.m.).
Supplementary Figure 5 Effects of central injection of TNF or bacterial infection on the release of fatty acids and leptin.
(a) Serum unconjugated fatty acids were measured for mice infected with Listeria monocytogenes (LM) or mice that received hypothalamic third-ventricle injection of TNF (10 pg) (T) or vehicle (V) as described in Figs. 1 and 2. Data represent n = 6–8 mice per group. (b–e) Standard C57BL/6 mice received TNF (10 pg) (+) vs. vehicle (–) injection in the third ventricle (b) as described in Fig. 1, or were intravenously injected with bacteria LM (+) vs. vehicle (–) (c), or co-treated with TNF antagonist WP9QY (W) and LM (+) vs. vehicle (–) injection (c,d) as described in Fig. 2, or injected with lentiviral TNFR1 and TNFR2 shRNA (T-s) vs. lentiviral nontargeting (scramble) shRNA (C-s) with LM (+) or without LM (–) infection (e), and following these treatments at the same time points as described in Fig. 1 to 3, blood samples were collected for measuring serum concentrations of leptin (n = 5 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA and Tukey’s post-hoc test); All results (mean) reflect two to three independent experiments with similar results (error bars, s.e.m.).
Supplementary Figure 6 Lipolysis mediates the effects of leptin on lymphocytes.
C57BL/6 mice were intraperitoneally pre-injected with cerulenin (Cer) (+) vs. vehicle (–) on the day before other treatments, and then this treatment continued daily for 3 d together with daily intraperitoneal injections of leptin (1.0 mg/kg) (Lep) (+) or vehicle (–), before tissues were harvested for flow cytometry. Data show numbers of T cells (CD3+) (a,e), CD4+ T cells (CD3+CD4+) (b,f), CD8+ T cells (CD3+CD8+) (c,g) and B cells (B220+) (d,h) per gram of epididymal fat (a–d) and spleen (e–h). *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA and Tukey’s post-hoc test); n = 4–5 mice per group. All results (mean) reflect two to three independent experiments with similar results (error bars, s.e.m.).
Supplementary Figure 7 Coordination of palmitate and leptin in increasing lymphocytes.
C57BL/6 mice were intravenously injected with leptin neutralizing antibody (2.5 mg/kg) (Ab) (+) vs. vehicle (–), and from day 2, they received daily intraperitoneal injections of palmitic acid (PA) (+) or vehicle (–) for 3 d before tissues were harvested for flow cytometry. Data show numbers of T cells (CD3+) (a,e), CD4+ T cells (CD3+CD4+) (b,f), CD8+ T cells (CD3+CD8+) (c,g) and B cells (B220+) (d,h) per gram of epididymal fat (a–d) and spleen (e–h). *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA and Tukey’s post-hoc test); n = 4–5 mice per group. Results (mean) reflect two to three independent experiments with similar results (error bars, s.e.m.).
Supplementary Figure 8 Conceptual model of this study.
Brain action of TNF is required to initiate the increases of peripheral lymphocytes, and this effect is mediated critically by hypothalamic TNF pathway. This function of hypothalamic TNF in initiating adaptive immunity is induced through hypothalamus-induced lipolysis in cooperation with release of other adipose factors such as leptin. These understanding suggest a conceptual point that the hypothalamus–fat axis plays a role in rapidly linking innate immune signal to adaptive immune responses.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 930 kb)
Rights and permissions
About this article
Cite this article
Kim, M., Yan, J., Wu, W. et al. Rapid linkage of innate immunological signals to adaptive immunity by the brain-fat axis. Nat Immunol 16, 525–533 (2015). https://doi.org/10.1038/ni.3133
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3133
This article is cited by
-
Hypothalamic administration of sargahydroquinoic acid elevates peripheral thermogenic signaling and ameliorates high fat diet-induced obesity through the sympathetic nervous system
Scientific Reports (2021)
-
An in vivo brain–bacteria interface: the developing brain as a key regulator of innate immunity
npj Regenerative Medicine (2020)
-
Reversal of prolonged obesity-associated cerebrovascular dysfunction by inhibiting microglial Tak1
Nature Neuroscience (2020)
-
When the Nervous System Turns Skeletal Muscles into Bones: How to Solve the Conundrum of Neurogenic Heterotopic Ossification
Current Osteoporosis Reports (2020)
-
Central s-resistin deficiency ameliorates hypothalamic inflammation and increases whole body insulin sensitivity
Scientific Reports (2018)