Abstract
The paradigm that macrophages that reside in steady-state tissues are derived from embryonic precursors has never been investigated in the intestine, which contains the largest pool of macrophages. Using fate-mapping models and monocytopenic mice, together with bone marrow chimera and parabiotic models, we found that embryonic precursor cells seeded the intestinal mucosa and demonstrated extensive in situ proliferation during the neonatal period. However, these cells did not persist in the intestine of adult mice. Instead, they were replaced around the time of weaning by the chemokine receptor CCR2–dependent influx of Ly6Chi monocytes that differentiated locally into mature, anti-inflammatory macrophages. This process was driven largely by the microbiota and had to be continued throughout adult life to maintain a normal intestinal macrophage pool.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
08 September 2014
In the version of this article initially published online, the vertical axis of the bottom right graph in Figure 3h was incorrect. It should read "F4/80loCD11b+ cells (×105)." Also, in the Online Methods, the first sentence of the third subsection ("Generation of bone marrow chimeras") identifies the donor mice incorrectly. It should read "...BM cells from CD45.2+ wild-type mice and CD45.1+ Ccr2-/- mice...." The errors have been corrected for the print, PDF and HTML versions of this article.
References
Davies, L.C., Jenkins, S.J., Allen, J.E. & Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 14, 986–995 (2013).
van Furth, R. et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull. World Health Organ. 46, 845–852 (1972).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210, 1977–1992 (2013).
Ajami, B., Bennett, J.L., Krieger, C., Tetzlaff, W. & Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543 (2007).
Lee, S.H., Starkey, P.M. & Gordon, S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J. Exp. Med. 161, 475–489 (1985).
Bain, C.C. & Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 260, 102–117 (2014).
Grimm, M.C. et al. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J. Gastroenterol. Hepatol. 10, 387–395 (1995).
Platt, A.M., Bain, C.C., Bordon, Y., Sester, D.P. & Mowat, A.M. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J. Immunol. 184, 6843–6854 (2010).
Varol, C. et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512 (2009).
Rivollier, A., He, J., Kole, A., Valatas, V. & Kelsall, B.L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155 (2012).
Bain, C.C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Tamoutounour, S. et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42, 3150–3166 (2012).
Qian, B.-Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Kennedy, D.W. & Abkowitz, J.L. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood 90, 986–993 (1997).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE 5, e13693 (2010).
Serbina, N.V. & Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Ginhoux, F. et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 (2009).
Davies, L.C. et al. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur. J. Immunol. 41, 2155–2164 (2011).
Ajami, B., Bennett, J.L., Krieger, C., McNagny, K.M. & Rossi, F.M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Liu, K. et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 8, 578–583 (2007).
Hill, D.A. et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 3, 148–158 (2010).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Bogunovic, M. et al. Origin of the lamina propria dendritic cell network. Immunity 31, 513–525 (2009).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013).
Jakubzick, C. et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610 (2013).
Chorro, L. et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 206, 3089–3100 (2009).
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 (2007).
Epelman, S., Lavine, K.J. & Randolph, G.J. Origin and functions of tissue macrophages. Immunity 41, 21–35 (2014).
Smythies, L.E. et al. Mucosal IL-8 and TGF-β recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J. Leukoc. Biol. 80, 492–499 (2006).
Ghigo, C. et al. Multicolor fate mapping of Langerhans cell homeostasis. J. Exp. Med. 210, 1657–1664 (2013).
Ueda, Y. et al. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int. Immunol. 22, 953–962 (2010).
Niess, J.H. & Adler, G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J. Immunol. 184, 2026–2037 (2010).
Müller, A.J. et al. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe 11, 19–32 (2012).
Nagashima, R., Maeda, K., Imai, Y. & Takahashi, T. Lamina propria macrophages in the human gastrointestinal mucosa: their distribution, immunohistological phenotype, and function. J. Histochem. Cytochem. 44, 721–731 (1996).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Boring, L. et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C–C chemokine receptor 2 knockout mice. J. Clin. Invest. 100, 2552–2561 (1997).
Benz, C. et al. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J. Exp. Med. 205, 1187–1199 (2008).
Bain, C.C. & Mowat, A.M. CD200 receptor and macrophage function in the intestine. Immunobiology 217, 643–651 (2012).
Acknowledgements
We thank D. Vaughan for help with flow cytometry; B. McColl (Roslin Institute, Edinburgh) for the CCR2-reporter mice; and V. Cerovic for critical reading of the manuscript. Supported by The Wellcome Trust (C.C.B., A.M.M. and C.L.S.), the Medical Research Council UK (C.C.B., A.M.M. and C.L.S.), Conacyt (A.B.-B.), Tenovus Scotland (A.B.-B.), the EE-ASI European Collaborative Research Project (S.H. and B.M.) and the Agence National de Recherche (S.H. and B.M.).
Author information
Authors and Affiliations
Contributions
C.C.B. designed, performed and analyzed experiments and wrote the manuscript; A.B.-B. designed, performed and analyzed experiments; C.L.S. performed experiments; E.G.P., F.G., S.H., B.M., L.C.O. and D.A. provided mice and reagents; and A.M.M. designed and supervised the studies and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
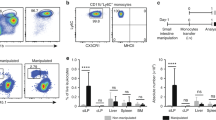
Supplementary Figure 1 Phenotypic characterization of colonic macrophages and Kupffer cells in neonatal and adult mice.
(a) Absolute numbers of total live CD45+ cells obtained from enzymatic digests of colon from newborn (day 2), day 7, day 14, day 21 and 7-8 week old adult CX3CR1+/gfp mice. Data were obtained from 3-6 individual mice per group. (b) Gating strategy used to identify F4/80+ CD11b+ cells amongst colon digests. Cell aggregates and dead cells were excluded on the basis of FSC and 7-AAD staining respectively. Leukocytes were then selected by their expression of CD45, before granulocytes were excluded by gating out SiglecF+ eosinophils and Ly6G+ neutrophils. Mucosal DC were excluded on the basis of their high levels of CD11c expression and lack of F4/80. (c) Expression of CD11c by F4/80+ CD11b+ colonic LP cells from 2 day old mice (black line) and adult mice (green line), and by total CD45+ leukocytes from adult colon (red line). (d) Quantitation by qRT-PCR of mRNA for CD163 by FACS-purified F4/80hi LP cells from resting colon of newborn (day 0-1) or adult mice, and CSF-1 generated BM-derived macrophages (BMM). Results are shown as mean expression relative to cyclophilin A (CPA), using the 2-ΔdC(t), with BMM set to 1. (e) Expression of MerTK by F4/80+ CD11b+ cells from colon of 2 day old mice and adult mice, and by F4/80hi CD11blo liver Kupffer cells. (f) Expression of CX3CR1-GFP and CD64 by F4/80hi CD11blo (red line) and F4/80lo CD11b+ (blue line) cells from colon of 2 day old CX3CR1-GFP reporter mice. (g) Gating strategy used to identify total F4/80+ CD11b+ cells amongst liver digests. Cell aggregates and dead cells were excluded on the basis of FSC and 7-AAD staining. CD45+ leukocytes were selected and granulocytes excluded by gating out SiglecF+ eosinophils and Ly6G+ neutrophils. Liver DC were excluded on the basis of their high levels of CD11c and MHCII expression.
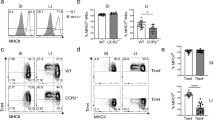
Supplementary Figure 2 Role of CCR2 in the development and phenotype of colonic macrophages.
(a) Representative expression of Ly6C and MHCII expression by total F4/80+ CD11b+ colonic LP cells from newborn (day 0-1), day 7, day 14 and day 21 Ccr2+/+ and Ccr2–/– mice. (b) Absolute numbers of F4/80+ CD11b+ Ly6ChiMHCII-, Ly6C+MHCII+ and Ly6C- cells per colon of newborn (day 0-1), day 7, day 14, day 21 and 7-8 week old adult Ccr2+/+ (filled circles) and Ccr2–/– mice (open circles). Data pooled from two independent time course experiments with a total of 6 mice (newborn (day 0-1), day 7, 14 and 21 time points) or 9 mice (adult time point). * P<0.05, *** P<0.01, *** P<0.001 (Student’s t-test). (c) Representative MHCII expression by F4/80+ CD11b+ Ly6C– cells from newborn (day 0-1), day 7, day 14, day 21 and 7-8 week old adult Ccr2+/+ (filled histogram) and Ccr2–/– mice (open histogram). (d) Representative expression of F4/80 and CD11b by live CD45+ SiglecF– Ly6G– CD11clo colonic LP cells (top panels), and Ly6C and MHCII expression by total F4/80+ CD11b+ cells (bottom panels) from 9-12 month old Ccr2+/+ and Ccr2–/– mice. Scatter plots show the absolute numbers of Ly6ChiMHCII–, Ly6C+MHCII+ and Ly6C– subsets amongst F4/80+ CD11b+ cells per colon of 9-12 month old Ccr2+/+ (filled circles) and Ccr2–/– mice (open circles). Data from one experiment with 5-6 mice per group. *** P<0.001 (Student’s t-test).
Supplementary Figure 3 Proliferative capacity of colonic myeloid cells.
(a) Mean proportion of Ki67+ cells amongst the MHCII– and MHCII+ fractions of the total F4/80+ CD11b+ Ly6C– population from colon of newborn (day 3), 2 week, 3 week, 5 week and 9 week old CX3CR1+/gfp mice. (b) F4/80 and CD11b expression profile of Ki67+ Ly6C– colonic LP cells (green) overlaid on that of the total macrophage gate (grey contour). (c) Mean proportion of Ki67+ Ly6C– cells bearing the F4/80hi CD11blo signature in colon of newborn (day 3), 2 week, 3 week, 5 week and 9 week old CX3CR1+/gfp mice. (d, e) MFI of F4/80 (d) and CD11b (e) expression by Ki67+ cells amongst total F4/80+ CD11b+ Ly6C– colonic cells from newborn (day 3), 2 week, 3 week, 5 week and 9 week old CX3CR1+/gfp mice. (f, g) Analysis of Ki67 expression by CD11chi F4/80– DC (f) and F4/80+SSChi eosinophils (g) in colon of (day 3), 2 week, 3 week, 5 week and 9 week old CX3CR1+/gfp mice. (h) Proportion of Ki67+ cells as a percentage of total F4/80+SSChi eosinophils in colon at each time point. Data are pooled from two independent experiments with 4-7 mice at each time point.
Supplementary Figure 4 Effects of broad-spectrum antibiotic treatment on cells of the ‘monocyte waterfall’ in the colon of adult mice.
Absolute numbers of Ly6ChiMHCII– (a), Ly6C+MHCII+ (b) and Ly6C– (c) subsets amongst F4/80+ CD11b+ cells per colon of CX3CR1+/gfp reporter mice that had received an antibiotic cocktail of ampicillin, neomycin, metrodinazole and vancomycin for two weeks (ABX) compared with control mice, which received sweetened water. Data from one of two independent experiments with 6 mice per group. * P<0.05 (Mann Whitney).
Supplementary Figure 5 Proposed model of age-dependent changes in the ontogeny and differentiation of colonic macrophages.
In foetal and newborn colon, essentially all mφ are derived from the yolk sac and/or foetal liver (red). These mφ have the signature of embryonically derived mφ, expressing high levels of F4/80 and low levels of CD11b. They have some similarities to their adult counterparts, expressing high levels of CX3CR1, being avidly phagocytic and producing IL10 constitutively. Unlike adult intestinal mφ however, they are almost exclusively MHCII– and produce lower levels of IL10. Their numbers are maintained in a CCR2-independent manner by high levels of in situ proliferation. It seems likely that the main function of mφ at this time is in removal of dying epithelial cells and tissue remodelling, and that their properties are conditioned by factors in the tissue environment itself.
Around 2-3 weeks of life, this proliferative capacity diminishes and the number of mφ with the embryonic signature decreases dramatically. This is paralleled by the full development of the microbiota and the appearance of the ‘monocyte waterfall’, in which Ly6Chi monocytes enter the intestinal mucosa in a CCR2-dependent manner and differentiate into mature macrophages through a series of short-lived intermediaries. The resulting cells are CX3CR1hiF4/80hi MHCIIhi CD11c+ and produce copious amounts of IL10 constitutively and are highly phagocytic, but are hyporesponsive to eg TLR ligation. The CCR2-dependent recruitment of Ly6Chi monocytes is partly driven by the microbiota and this process is essential for maintaining the macrophage pool in adult intestine. Continuous supply of monocytes that differentiate under the influence of the local environment allows intestinal mφ to fulfil physiological functions such as tissue remodelling and clearance of commensal bacteria without provoking inflammation, while at the same time sampling for pathogenic invaders.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 2852 kb)
Rights and permissions
About this article
Cite this article
Bain, C., Bravo-Blas, A., Scott, C. et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15, 929–937 (2014). https://doi.org/10.1038/ni.2967
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2967
This article is cited by
-
The roles of tissue resident macrophages in health and cancer
Experimental Hematology & Oncology (2024)
-
LSD1 drives intestinal epithelial maturation and controls small intestinal immune cell composition independent of microbiota in a murine model
Nature Communications (2024)
-
Systemically delivered adipose stromal vascular fraction mitigates radiation-induced gastrointestinal syndrome by immunomodulating the inflammatory response through a CD11b+ cell-dependent mechanism
Stem Cell Research & Therapy (2023)
-
Colitis-associated carcinogenesis: crosstalk between tumors, immune cells and gut microbiota
Cell & Bioscience (2023)
-
Macrophage polarization in inflammatory bowel disease
Cell Communication and Signaling (2023)