Abstract
The number of Foxp3+ regulatory T cells (Treg cells) must be tightly controlled for efficient suppression of autoimmunity with no impairment of normal immune responses. Here we found that the adaptor TRAF3 was intrinsically required for restraining the lineage determination of thymic Treg cells. T cell–specific deficiency in TRAF3 resulted in a two- to threefold greater frequency of Treg cells, due to the more efficient transition of precursors of Treg cells into Foxp3+ Treg cells. TRAF3 dampened interleukin 2 (IL-2) signaling by facilitating recruitment of the tyrosine phosphatase TCPTP to the IL-2 receptor complex, which resulted in dephosphorylation of the signaling molecules Jak1 and Jak3 and negative regulation of signaling via Jak and the transcription factor STAT5. Our results identify a role for TRAF3 as an important negative regulator of signaling via the IL-2 receptor that affects the development of Treg cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Burchill, M.A. et al. Linked TCR and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28, 112–121 (2008).
Lio, C.W. & Hsieh, C.S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Malek, T.R. The biology of IL-2. Annu. Rev. Immunol. 26, 453–479 (2008).
Johnston, J.A., Bacon, C.M., Riedy, M.C. & O'Shea, J.J. Signaling by IL-2 and related cytokines: Jaks, STATs, and relationship to immunodeficiency. J. Leukoc. Biol. 60, 441–452 (1996).
Minami, Y. & Taniguchi, T. IL-2 signaling: recruitment and activation of multiple protein tyrosine kinases by the components of the IL-2 receptor. Curr. Opin. Cell Biol. 7, 156–162 (1995).
Burchill, M.A., Yang, J., Vogtenhuber, C., Blazar, B.R. & Farrar, M.A. IL-2Rβ dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178, 280–290 (2007).
Yao, Z. et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109, 4368–4375 (2007).
Burns, L.A., Karnitz, L.M., Sutor, S.L. & Abraham, R.T. IL-2-induced tyrosine phosphorylation of p52shc in T lymphocytes. J. Biol. Chem. 268, 17659–17661 (1993).
Zhou, Y.J. et al. Hierarchy of protein tyrosine kinases in IL-2 signaling: activation of Syk depends on Jak3; however, neither Syk nor Lck is required for IL-2-mediated STAT activation. Mol. Cell. Biol. 20, 4371–4380 (2000).
Sporri, B., Kovanen, P.E., Sasaki, A., Yoshimura, A. & Leonard, W.J. JAB/SOCS1/SSI-1 is an IL-2-induced inhibitor of IL-2 signaling. Blood 97, 221–226 (2001).
Yu, C.R. et al. SOCS3 regulates proliferation and activation of T-helper cells. J. Biol. Chem. 278, 29752–29759 (2003).
Migone, T.S. et al. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the IL-2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc. Natl. Acad. Sci. USA 95, 3845–3850 (1998).
Simoncic, P.D., Lee-Loy, A., Barber, D.L., Tremblay, M.L. & McGlade, C.J. The T cell protein tyrosine phosphatase is a negative regulator of Jaks 1 and 3. Curr. Biol. 12, 446–453 (2002).
Ibarra-Sánchez, M.J. et al. The T-cell protein tyrosine phosphatase. Semin. Immunol. 12, 379–386 (2000).
You-Ten, K.E. et al. Impaired bone marrow microenvironment and immune function in TCPTP-deficient mice. J. Exp. Med. 186, 683–693 (1997).
Wiede, F. et al. TCPTP attenuates T cell signaling to maintain tolerance in mice. J. Clin. Invest. 121, 4758–4774 (2011).
Häcker, H. et al. Specificity in TLR signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207 (2006).
Hildebrand, J.M. et al. Roles of TRAF3 and TRAF5 in immune cell functions. Immunol. Rev. 244, 55–74 (2011).
Yi, Z., Lin, W.W., Stunz, L.L. & Bishop, G.A. Roles for TNF-receptor associated factor 3 (TRAF3) in lymphocyte functions. Cytokine Growth Factor Rev. 25, 147–156 (2014).
Bishop, G.A. The many faces of TRAF molecules in immune regulation. J. Immunol. 191, 3483–3485 (2013).
Xie, P., Kraus, Z.J., Stunz, L.L., Liu, Y. & Bishop, G.A. TRAF3 is required for T cell-mediated immunity and TCR/CD28 signaling. J. Immunol. 186, 143–155 (2011).
Yi, Z., Stunz, L.L. & Bishop, G.A. TRAF3 plays a key role in development and function of invariant natural killer T cells. J. Exp. Med. 210, 1079–1086 (2013).
Chang, J.H. et al. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J. Exp. Med. 211, 137–151 (2014).
Bailey-Bucktrout, S.L. & Bluestone, J.A. Regulatory T cells: stability revisited. Trends Immunol. 32, 301–306 (2011).
Josefowicz, S.Z. & Rudensky, A. Control of regulatory T cell lineage commitment and maintenance. Immunity 30, 616–625 (2009).
Floess, S. et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 5, e38 (2007).
Thornton, A.M. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441 (2010).
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209, 1713–1722 (2012).
Weiss, J.M. et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209, 1723–1742 (2012).
Hofmann, J., Mair, F., Greter, M., Schmidt-Supprian, M. & Becher, B. NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J. Exp. Med. 208, 1917–1929 (2011).
Murray, S.E. Cell-intrinsic role for NIK in peripheral maintenance but not thymic development of Foxp3+ regulatory T cells in mice. PLoS ONE 8, e76216 (2013).
Moran, A.E. et al. TCR signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Lal, G. & Bromberg, J.S. Epigenetic mechanisms of regulation of Foxp3 expression. Blood 114, 3727–3735 (2009).
Sakaguchi, S., Fukuma, K., Kuribayashi, K. & Masuda, T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 161, 72–87 (1985).
Kayagaki, N. et al. DUBA: a deubiquitinase that regulates type I interferon production. Science 318, 1628–1632 (2007).
Bautista, J.L. et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 10, 610–617 (2009).
Yu, A., Zhu, L., Altman, N.H. & Malek, T.R. A low IL-2R signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30, 204–217 (2009).
Bensinger, S.J. et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 172, 5287–5296 (2004).
Walsh, P.T. et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+CD25+ Tregs. J. Clin. Invest. 116, 2521–2531 (2006).
Zheng, Y. et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 445, 936–940 (2007).
Motegi, H., Shimo, Y., Akiyama, T. & Inoue, J. TRAF6 negatively regulates the Jak1-Erk pathway in IL-2 signaling. Genes Cells 16, 179–189 (2011).
van Vliet, C. et al. Selective regulation of TNF-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat. Immunol. 6, 253–260 (2005).
Lin, W.W., Hildebrand, J.M. & Bishop, G.A. A complex relationship between TRAF3 and non-canonical NF-κB2 activation in B lymphocytes. Front Immunol. 4, 477 (2013).
He, J.Q., Saha, S.K., Kang, J.R., Zarnegar, B. & Cheng, G. Specificity of TRAF3 in its negative regulation of the non-canonical NF-κB pathway. J. Biol. Chem. 282, 3688–3694 (2007).
Xie, P., Stunz, L.L., Larison, K.D., Yang, B. & Bishop, G.A. TRAF3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity 27, 253–267 (2007).
Holst, J. et al. Generation of T-cell receptor retrogenic mice. Nat. Protoc. 1, 406–417 (2006).
Ostanin, D.V. et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G135–G146 (2009).
Tiganis, T., Bennett, A.M., Ravichandran, K.S. & Tonks, N.K. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol. Cell. Biol. 18, 1622–1634 (1998).
Acknowledgements
We thank F. Sutterwala (University of Iowa) for Rag1−/− mice; A. Rudensky (Memorial Sloan-Kettering Cancer Center) and T.J. Waldschmidt (University of Iowa) for Foxp3-GFP mice; A. Schlueter (University of Iowa) for OT-II mice; R. Schreiber (University of Washington at St. Louis) and D. Parker (Oregon Health Science University) for Nik−/− mice; N. Tonks (Cold Spring Harbor Laboratory) for plasmids encoding the 45-kDa and 48-kDa forms of TCPTP and the substrate-trapping mutant of each; and J. Colgan for advice in making retrogenic BM chimeras. Supported by the National Cancer Institute of the US National Institutes of Health (P30CA086862), the US National Institutes of Health (AI28847 to G.A.B., and 5T32AI007260-27 for support for Z.Y.) and the US Department of Veterans Affairs (G.A.B.). This work was based on work supported by the Office of Research and Development of the Veterans Health Administration of the US Department of Veterans Affairs.
Author information
Authors and Affiliations
Contributions
Z.Y. designed and did experiments, analyzed data and wrote the manuscript; W.W.L. and L.L.S. did experiments, provided input for data interpretation and edited the manuscript; and G.A.B. conceived of the research, directed the study, interpreted data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
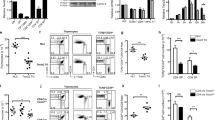
Supplementary Figure 1 TRAF3 restrains the development of Treg cells.
(a) Frequency of Foxp3+ cells in CD4+ T cells in control littermate (CLM) and Cd4CreTraf3flox/flox (T-Traf3−/−) mice. Data presented are representative of data from 6-10 mice. MLN=mesenteric lymph nodes, ILN=inguinal lymph nodes, BLN=brachial lymph nodes, ALN=axillary lymph nodes, PP=Peyer’s Patch.
(b) Frequency of Foxp3+ cells in CD4+ T cells in CLM and LckCreTraf3flox/flox mice. Data shown are representative of data from six mice.
Supplementary Figure 2 TRAF3 deficiency does not alter the properties of Treg cells.
(a) CD4+CD25+ Treg cells were isolated from spleen and stimulated with anti-CD3 and CD28 Abs with/without 50U rmIL-2/ml for 72 hrs. Mean fluorescence intensity (MFI) of Foxp3 is shown. Error bars are mean values ± SEM of three replicate wells. Data represent one of three experiments. NS, not significant.
(b) Freshly isolated splenocytes were stained for Annexin V, CD4 and Foxp3. Numbers represent percentage of Annexin V positive cells in CD4+Foxp3+ cells. Data shown are representative of data obtained from six mice per group.
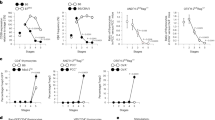
Supplementary Figure 3 T cell activation of NF-κB2 does not account for the higher frequency of Treg cells in T-Traf3−/− mice.
(a) Flow cytometric analysis of Treg cells in WT, T-Traf3−/−, Nik−/− and Nik−/− T-Traf3−/− mice. Thymocytes were stained for CD4, CD8 and Foxp3. Numbers represent percentage of Foxp3+ cells in CD4 single positive cells. Data are shown for one mouse representative of six mice from each group.
(b) Flow cytometric analysis of Treg cells in bone marrow chimeric mice. In one set of experiments, bone marrow cells from Nik−/− and Nik−/− T-Traf3−/− mice were transferred into sublethally irradiated Rag1−/− mice. In another set of experiments, bone marrow cells from Rag1−/− mice were mixed with bone marrow cells from WT, T-Traf3−/−, Nik−/− and Nik−/− T-Traf3−/− mice at a 10:1 ratio and transferred into sublethally irradiated Rag1−/−mice. Thymocytes were stained for CD4, CD8 and Foxp3. Numbers represent percentage of Foxp3+ cells in CD4 single positive cells. Data are from a representative mouse of four from each group.
Supplementary Figure 4 Thymic selection is not altered in T-Traf3−/− mice.
(a) Thymocytes from CLM and T-Traf3−/− mice were stained for CD4 and CD8. Percentage (left panel) and numbers (right panel) of different populations are shown. (DN, double negative; DP, double positive). Error bars are mean values ± SEM of 6 mice per group.
(b) and (c) Thymocytes from CLM OTII and T-Traf3−/− OTII mice were stained for CD4, CD8 and Vβ5. Left panel in (b) shows percentages of different populations. Right panel in (b) shows cell numbers of DP and CD4 single positive cells. Percentage of Vβ5 positive cells in CD4 single positive cells is shown in (c). Data are representative of one of six mice from each group.
(d) Flow cytometric analysis of Nur77 expression in thymocytes in CLM and T-Traf3−/− mice. MFI of Nur77 expression in CD4+Foxp3+ (Treg), CD4+Foxp−CD25+ (precursor) and CD4+Foxp−CD25− (Tcon) cells are shown. Error bars are mean values ± SEM of 6 mice.
(e) Flow cytometric analysis of precursor of Treg cells in CLM or T-Traf3−/− mice. Thymocytes were stained for CD4, CD8, Foxp3, CD25 and GITR. CD4+CD8−Foxp3− cells were gated. Numbers represent percentage of CD25+GITR+ cells. Data are presented from a representative of six mice in each group.
Supplementary Figure 5 The transition from precursor to Treg cell is increased due to enhanced IL-2 signaling in the absence of TRAF3.
(a) and (b) Sorted precursors of Treg cells (CD4+CD8-Foxp3GFP−CD25+ cells) from thymi were stimulated with 50U IL-2 for 20 min. p-Erk (a) and p-Akt (b) were stained by intracellular staining. Data represent one of three experiments.
(c) Flow cytometric analysis of IL-2 receptors on precursors of Treg cells. Thymocytes were stained for CD4, CD8, Foxp3, CD25, CD122 and CD132. The expression levels of CD25, CD122 and CD132 on precursors of Treg cells are shown. Data are from a representative of six mice in each group.
Supplementary Figure 6 TRAF3 restrains IL-2 signaling in conventional CD4+CD25− T cells.
(a, b) CD4+CD25- T cells were purified from splenocytes. 1x107 cells were stimulated with 500U/ml IL-2 for 1 hr. Nuclei were lysed, sonicated and subjected to IP with anti-STAT5 Ab. DNA was extracted from precipitates and relative amounts of the STAT5 target genes, IL-2Rα (a) and Cis (b) were quantified by real-time PCR. Values were normalized to corresponding input DNA and are expressed as fold enrichment relative to untreated control. * p<0.05.
(c, d) CD4+Foxp3GFP+ and CD4+Foxp3GFP− cells were sorted from spleen in CLM and Foxp3GFP T-Traf3−/–- mice. CD4+Foxp3GFP+ Treg cells were stimulated with 50U/ml rmIL-2 for 15 min (c). CD4+Foxp3GFP− cells were stimulated with 500U/ml rmIL-2 (d). p-STAT5, β-actin and TRAF3 were measured by Western blot.
(e) CD4+CD25+ Treg cells and CD4+CD25− T conventional cells were sorted from splenocytes. Whole cell lysates were blotted for TRAF3 and β-actin. Data represent one of two experiments.
Supplementary Figure 7 Interactions between TRAF3 and TCPTP restrain signaling by IL-2 and IFN-γ.
(a) Human CD4+ T cells were transfected with Tcptp siRNA and treated with 5ng/ml rhIL-2. Western blots of cell lysates probed for p-STAT5, TCPTP and β-actin are shown.
(b) CD4+CD25− T cells isolated from mouse splenocytes were stimulated with 500U/ml rmIL-2. CD122 was immunoprecipitated from cell lysates. The IPs were blotted for Jak1, Jak3, SHP-1 and CD122. Whole cell lysates (WCL) were blotted for Jak1.
(c) CD4+CD25− T cells isolated from mouse splenocytes were stimulated with 10ng/ml IFN-γ. Whole cell lysates were blotted for p-STAT1, STAT1, β-actin and TRAF3.
(d) Plasmids encoding HA-tagged ubiquitin, FLAG-tagged Traf3 and Tcptp were transfected into 293 cells. Denatured cell lysates were subjected to immunoprecipitation with anti-TCPTP Ab. IPs were blotted for HA, TCPTP and TRAF3. Whole cell lysates were blotted for TCPTP, FLAG and β-actin. Data represent one of two experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 58941 kb)
Rights and permissions
About this article
Cite this article
Yi, Z., Lin, W., Stunz, L. et al. The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL-2. Nat Immunol 15, 866–874 (2014). https://doi.org/10.1038/ni.2944
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2944
This article is cited by
-
IL-2 immunotherapy for targeting regulatory T cells in autoimmunity
Genes & Immunity (2023)
-
TRAF3 regulates the oncogenic proteins Pim2 and c-Myc to restrain survival in normal and malignant B cells
Scientific Reports (2019)
-
TRAF Molecules in Inflammation and Inflammatory Diseases
Current Pharmacology Reports (2018)
-
TRAF3 enhances TCR signaling by regulating the inhibitors Csk and PTPN22
Scientific Reports (2017)
-
Mechanisms and consequences of Jak–STAT signaling in the immune system
Nature Immunology (2017)