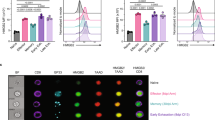
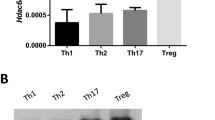
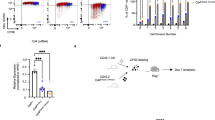
Abstract
Molecular mechanisms that maintain lineage integrity of helper T cells are largely unknown. Here we show histone deacetylases 1 and 2 (HDAC1 and HDAC2) as crucial regulators of this process. Loss of HDAC1 and HDAC2 during late T cell development led to the appearance of major histocompatibility complex (MHC) class II–selected CD4+ helper T cells that expressed CD8-lineage genes such as Cd8a and Cd8b1. HDAC1 and HDAC2–deficient T helper type 0 (TH0) and TH1 cells further upregulated CD8-lineage genes and acquired a CD8+ effector T cell program in a manner dependent on Runx-CBFβ complexes, whereas TH2 cells repressed features of the CD8+ lineage independently of HDAC1 and HDAC2. These results demonstrate that HDAC1 and HDAC2 maintain integrity of the CD4 lineage by repressing Runx-CBFβ complexes that otherwise induce a CD8+ effector T cell–like program in CD4+ T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Change history
06 June 2014
In the version of this article initially published, Lisa Göschl's surname was spelled incorrectly. The error has been corrected in the HTML and PDF versions of the article.
References
Brunmeir, R., Lagger, S. & Seiser, C. Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int. J. Dev. Biol. 53, 275–289 (2009).
Haberland, M., Montgomery, R.L. & Olson, E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 10, 32–42 (2009).
Villagra, A., Sotomayor, E.M. & Seto, E. Histone deacetylases and the immunological network: implications in cancer and inflammation. Oncogene 29, 157–173 (2010).
Shakespear, M.R., Halili, M.A., Irvine, K.M., Fairlie, D.P. & Sweet, M.J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 32, 335–343 (2011).
Hancock, W.W., Akimova, T., Beier, U.H., Liu, Y. & Wang, L. HDAC inhibitor therapy in autoimmunity and transplantation. Ann. Rheum. Dis. 71 (Suppl 2), i46–i54 (2012).
Grausenburger, R. et al. Conditional deletion of histone deacetylase 1 in T cells leads to enhanced airway inflammation and increased Th2 cytokine production. J. Immunol. 185, 3489–3497 (2010).
LeBoeuf, M. et al. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev. Cell 19, 807–818 (2010).
Montgomery, R.L., Hsieh, J., Barbosa, A.C., Richardson, J.A. & Olson, E.N. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc. Natl. Acad. Sci. USA 106, 7876–7881 (2009).
Wilting, R.H. et al. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J. 29, 2586–2597 (2010).
Yamaguchi, T. et al. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 24, 455–469 (2010).
Kasler, H.G. et al. Histone deacetylase 7 regulates cell survival and TCR signaling in CD4/CD8 double-positive thymocytes. J. Immunol. 186, 4782–4793 (2011).
Kasler, H.G. et al. Nuclear export of histone deacetylase 7 during thymic selection is required for immune self-tolerance. EMBO J. 31, 4453–4465 (2012).
Konkel, J.E. et al. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nat. Immunol. 12, 312–319 (2011).
Ellmeier, W., Sunshine, M.J., Losos, K., Hatam, F. & Littman, D.R. An enhancer that directs lineage-specific expression of CD8 in positively selected thymocytes and mature T cells. Immunity 7, 537–547 (1997).
Hostert, A. et al. A region in the CD8 gene locus that directs expression to the mature CD8 T cell subset in transgenic mice. Immunity 7, 525–536 (1997).
Hassan, H. et al. Cd8 enhancer E8I and Runx factors regulate CD8alpha expression in activated CD8+ T cells. Proc. Natl. Acad. Sci. USA 108, 18330–18335 (2011).
Glimcher, L.H., Townsend, M.J., Sullivan, B.M. & Lord, G.M. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat. Rev. Immunol. 4, 900–911 (2004).
Cruz-Guilloty, F. et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206, 51–59 (2009).
Rui, J., Liu, H., Zhu, X., Cui, Y. & Liu, X. Epigenetic Silencing of Cd8 Genes by ThPOK-Mediated Deacetylation during CD4 T Cell Differentiation. J. Immunol. 189, 1380–1390 (2012).
Jenkinson, S.R. et al. Expression of the transcription factor cKrox in peripheral CD8 T cells reveals substantial postthymic plasticity in CD4–CD8 lineage differentiation. J. Exp. Med. 204, 267–272 (2007).
Egawa, T. & Littman, D.R. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat. Immunol. 9, 1131–1139 (2008).
Wang, L. et al. The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity 29, 876–887 (2008).
Muroi, S. et al. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat. Immunol. 9, 1113–1121 (2008).
Taniuchi, I. et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 111, 621–633 (2002).
Dokmanovic, M., Clarke, C. & Marks, P.A. Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 5, 981–989 (2007).
Naoe, Y. et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J. Exp. Med. 204, 1749–1755 (2007).
Sato, T. et al. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity 22, 317–328 (2005).
Balasubramanyam, K., Swaminathan, V., Ranganathan, A. & Kundu, T.K. Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem. 278, 19134–19140 (2003).
Yagi, R. et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity 32, 507–517 (2010).
Yagi, R., Zhu, J. & Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 23, 415–420 (2011).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Ellmeier, W., Sunshine, M.J., Maschek, R. & Littman, D.R. Combined deletion of CD8 locus cis-regulatory elements affects initiation but not maintenance of CD8 expression. Immunity 16, 623–634 (2002).
He, X. et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature 433, 826–833 (2005).
Sun, G. et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat. Immunol. 6, 373–381 (2005).
Zhang, M., Zhang, J., Rui, J. & Liu, X. p300-mediated acetylation stabilizes the Th-inducing POK factor. J. Immunol. 185, 3960–3969 (2010).
Pozzesi, N. et al. Role of caspase-8 in thymus function. Cell Death Differ. 21, 226–233 (2014).
Ellmeier, W., Sunshine, M.J., Losos, K. & Littman, D.R. Multiple developmental stage-specific enhancers regulate CD8 expression in developing thymocytes and in thymus-independent T cells. Immunity 9, 485–496 (1998).
Sakaguchi, S. et al. The zinc-finger protein MAZR is part of the transcription factor network that controls the CD4 versus CD8 lineage fate of double-positive thymocytes. Nat. Immunol. 11, 442–448 (2010).
Bartl, S. et al. Identification of mouse histone deacetylase 1 as a growth factor-inducible gene. Mol. Cell. Biol. 17, 5033–5043 (1997).
Wisniewski, J.R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009).
Maurer, M. et al. Combining filter-aided sample preparation and pseudoshotgun technology to profile the proteome of a low number of early passage human melanoma cells. J. Proteome Res. 12, 1040–1048 (2013).
Bennett, K.L. et al. Proteomic analysis of human cataract aqueous humour: Comparison of one-dimensional gel LCMS with two-dimensional LCMS of unlabelled and iTRAQ(R)-labelled specimens. J. Proteomics 74, 151–166 (2011).
Colinge, J., Masselot, A., Giron, M., Dessingy, T. & Magnin, J. OLAV: towards high-throughput tandem mass spectrometry data identification. Proteomics 3, 1454–1463 (2003).
Breitwieser, F.P. et al. General statistical modeling of data from protein relative expression isobaric tags. J. Proteome Res. 10, 2758–2766 (2011).
Vogel, C. & Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232 (2012).
Acknowledgements
We thank W. Glaser for performing the microarray experiments, D. Printz for cell sorting, C. Humer for help with immunoblot analysis, E. Pfeiffer for help with irradiation, R. Bosselut (US National Cancer Institute, National Institutes of Health (NIH)) for the anti–Th-POK antibody and J. Zhu (US National Institute of Allergy and Infectious Diseases, NIH) for the GATA-3 vector. This study was funded by the Vienna Science and Technology Fund (WWTF) through project LS09-031 (to W.E. and C.S.). This work in the laboratory of W.E. was also supported by the Austrian Science Fund (FWF) and MedUni Vienna doctoral program (DK W1212) “Inflammation and Immunity” and by FWF projects (P19930, P23641 and I00698). The work in the laboratory of C.S. was supported by the FWF (P25807 and DKplus W1220) and the Genome Research in Austria project “Epigenetic Regulation of Cell Fate Decisions” (Federal Ministry of Science, Research and Economy, BMWFW). N.B. and S.S. are funded by the FWF (P24265 and P23669, respectively). L.G. has been supported from the Innovative Medicines Initiative Joint Undertaking under grant agreement 115142 (BTCure), resources of which are composed of financial contribution from the FP7 of the European Union and the European Federation of Pharmaceutical Industries and Associations companies' in kind contribution. T.E. is supported by NIH grant AI097244.
Author information
Authors and Affiliations
Contributions
N.B., R.T., C.S. and W.E. designed the research; N.B. and R.T. performed most of the experiments and analyzed the data; L.G., S.L. M.W., L.M. and H.H. performed some of the experiments and analyzed data; S.S. designed some of the research and analyzed data; M.A.M. performed ChIP assays and analyzed data; F.L. and W.S. analyzed microarray data, T.E. provided experimental data and reagents, I.T. and P.M. provided reagents and mice; K.L.B. and D.V. designed and performed the mass spectrometric experiments; F.P.B. and J.C. performed analysis of the proteomic data set and cross correlation with the microarray data; N.B., R.T., C.S. and W.E. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Characterization of T cells in HDAC2 cKO mice.
(a) Flow cytometry analysis of B220, TCRβ, CD4 and CD8α expression on splenocytes isolated from WT and HDAC2 cKO mice. (b) Flow cytometry analysis of CD4, CD8α, CD24 and TCRβ expression on WT and HDAC2 cKO thymocytes. The CD4 and CD8α plot to the right is gated on HSAloTCRβhi cells. (c) Flow cytometry showing intracellular HDAC2 expression levels in WT and HDAC2 cKO mice DN, DP, CD4SP and CD8SP thymocyte subsets and in splenic CD4+ and CD8+ T cells. CD4SP and CD8SP cells were gated on TCRβhi subsets. The dotted vertical line separates HDAC2-negative and HDAC2-positive cells. (d) Flow cytometry analysis of CD44 and CD62L expression on TCRβ+ splenic WT and HDAC2 cKO CD4+ and CD8+ T cells. (e) Flow cytometry analysis of CD25 and intracellular FoxP3 expression in splenic WT and HDAC2 cKO CD4+ T cells. (f) Flow cytometry analysis of intracellular HDAC1 and HDAC2 expression in splenic WT and HDAC2 cKO CD4+ and CD8+ T cells. (a,b,d-f) Numbers indicate the percentage of cells in the respective quadrants and regions. Data are representative of six (WT) and seven (HDAC2 cKO mice) mice (a,b,d), of three mice (c) and of two mice (e,f) analyzed in four (a,b,d) and in one (c,e,f) experiments.
Supplementary Figure 2 Characterization of T cell subsets in HDAC1-2 cKO mice.
(a) Flow cytometry analysis of TCRβ expression on WT CD4+ and CD8+ T cells and on HDAC1-2 cKO CD4+, CD8+ and CD4+CD8+ T cells. (b) Mean fluorescence intensity (MFI) of CD4 expression on WT CD4+ T cells and on HDAC1-2 cKO CD4+ and CD4+CD8+ T cells. Per experimental staining group, wild-type MFI CD4 was set as 1 and the relative expression HDAC1-HDAC2-deficient CD4+ and CD8+ T cells was calculated. (c) Flow cytometry analysis of CD25 and intracellular FoxP3 expression in CD4+ T cells from WT CD4+ T cells and in HDAC1-2 cKO CD4+ and CD4+CD8+ T cells (d) Percentages of CD44hi splenic WT CD4+ and CD8+ T cells and of HDAC1-2 cKO CD4+, CD8+ and CD4+CD8+ T cells. (e) Flow cytometry analysis of intracellular IFN-γ and TNF-α expression in splenic WT and HDAC1-2 cKO CD4+ and CD8+ T cells and in HDAC1-2 cKO CD4+CD8+ T cells activated ex vivo with PMA/ionomycin for 5 hours. (f) Flow cytometric bead assays showing IFN-γ, IL-4, TNFα and GM-CSF amount in the supernatant of WT and HDAC1-2 cKO CD4+ and CD8+ T cells and HDAC1-2 cKO CD4+CD8+ T cells activated with anti-CD3 and anti-CD28 for 24 hours. (g) Flow cytometry analysis showing CD62L, CD122, CD127, CCR7 and CD27 expression on naïve (CD44lo) and effector/memory (CD44hi) splenic WT and HDAC1-2 cKO CD4+ and CD8+ T cells. The expression of these markers on total HDAC1-2 cKO CD4+CD8+ splenocytes is shown as dashed line on top of each panel. (c,e,g) Numbers indicate the percentage of cells in the respective quadrant or regions. Data are representative (a,c,e,g) or show summary (b,d,f) of six mice (a), of seven mice (b), of two mice (c), of seven (WT) and eight (HDAC1-2 cKO) mice (d), of four mice (e), of two independent samples (f) and of six mice except for CD27 (two mice) (g) that were analyzed in three (a,b), in one (c) in four (d) and in two (e,f,g) independent experiments. (b) *P < 0.05 (Wilcoxon signed rank test). Mean ± SD is shown. (d) *P < 0.05 (unpaired two-tailed Mann-Whitney test). NS, not significant.
Supplementary Figure 3 T cell defects in HDAC1-2 cKO CD4+ T cells are cell-intrinsic, and CD8 expression in HDAC1-2 cKO CD4+ T cells is dependent on Cd8 enhancer E8I.
(a) Flow cytometry analysis of B220, TCRβ, CD4 and CD8α on splenocytes isolated from CD45.1+ chimeric mice that received either WT or HDAC1-2 cKO CD45.2+ BM cells that had been mixed at a 1:1 ratio with wild-type (WT, CD45.1+) BM cells. Cells were gated on CD45.1+ and CD45.2+ subsets. Flow cytometry analysis of CD4 and CD8α expression was performed on TCRβ+ splenocytes. (b) Flow cytometry analysis of CD154 and CD69 expression on WT CD4+ and CD8+ T cells and on HDAC1-2 cKO CD4+, CD8+ and CD4+CD8+ T cells activated with anti-CD3 and anti-CD28 for 12 hours. (c) PCR analysis of DNA isolated from WT CD4+ and CD8+ T cells and B220+ B cells, and from HDAC1-2 cKO CD4+, CD8+ and CD4+CD8+ T cells and B220+ B cells to detect deletion of Hdac1 and Hdac2. The lower panel shows a PCR analysis of DNA isolated from WT B cells mixed with either HDAC1 cKO or HDAC2 cKO CD4+ T cells at the indicated ratio to determine the sensitivity of the PCR assay. The size of the PCR fragments are for Hdac1: floxed (F): 567 bp; deleted (Δ): 535 bp; for Hdac2: floxed (F): 850bp; deleted (Δ): 670bp. (d) Flow cytometry analysis of CD4 and CD8α expression on E8I+/+WT and E8I−/−HDAC1-2 cKO CD4+ T cells activated with anti-CD3 and anti-CD28 for 48 hours. (a,d) Numbers in the plots indicate the percentage of cells in the respective quadrants and regions. (a-d) Data are representative of three (a) and two (b,d) mice per group analyzed in two independent experiments (a-d).
Supplementary Figure 4 Gene expression patterns in CD4 lineage T cells in the absence of HDAC1 and HDAC2.
(a) Gene expression profiles from WT CD4+ T cells and from HDAC1-2 cKO CD4+ and CD4+CD8+ T cells were determined using Agilent arrays. Data were analyzed using Genespring software. Scatter plots show HDAC1-2 cKO (Y-axis) versus WT (X-axis) CD4+ T cells. The scale on the X- and Y-axis indicate expression levels (log2). Numbers at the upper-right or lower-left corners show the number of genes up- or down-regulated (fold-difference ≥2, P≤0.05) in the absence of HDAC1 and HDAC2, respectively. (b) qRTPCR analysis showing Cd4, Cd8a and Cd8b1 expression (relative to Hprt1) in WT CD4+ and CD8+ T cells and in HDAC1-2 cKO CD4+, CD8+ and CD4+CD8+ T cells. Arbitrary units are shown. RNA samples were identical to the ones used for microarrays in (a). The summary of 3 independent experiments is shown. *P < 0.05, **P < 0.01 and ***P < 0.001 (unpaired two-tailed Student's t-test). Mean with SD is shown. (c) Agilent microarray expression data of selected genes in WT CD4+ T cells and HDAC1-2 cKO CD4+ and CD4+CD8+ T cells are shown. The expression levels in WT CD4+ T cells were set as 1 and the relative expression in the other subsets was calculated. The numbers indicate the relative expression value. Only one representative Agilent array probe per gene is shown. The following probes are shown: Runx3 (A_55_P2130895), Eomes (A_55_P2150717), Prf1 (A_55_P2000039), Zbtb7b (A_55_P2020348), Cd4 (A_55_P2013655), Cd8a A_52_P443334), Cd8b1 (A_55_P2154982). Data show the summary of three arrays per cell population. *P < 0.05 (unpaired two-tailed Student's t-test). (d) qRTPCR analysis showing Eomes, Runx3, Tbx21 and Zbtb7b expression in naïve WT CD4+ T cells and in naive HDAC1-2 cKO CD4+ and CD4+CD8+) T cells. Data show summary of 3 (for CD4+ T cells) and 2 (for HDAC1-2 cKO CD4+CD8+ T cells) independent biological replicas performed in two (CD4+ T cells) and one (CD4+CD8+ T cells) experiment(s).
Supplementary Figure 5 HDAC1-HDAC2 binding to CD8-lineage genes, the effect of GATA3 on MS-275−induced CD8 upregulation and a proteomic versus transcriptomic comparison.
(a) qRTPCR with primers specific for Cd8a, Cd8b1, Runx3 and Eomes promoter regions and for Cd8 enhancer E8I from chromatin of non-activated (N) and activated (A; anti-CD3 with anti-CD28 for 60 hours) wild-type CD4+ (left panel) and CD8+ (right panel) T cells immunoprecipitated with anti-HDAC1 or anti-HDAC2 antibodies (or IgG and IgM as control). Mean of 2 independent experiments is shown. Dots display the results of the individual experiments. One representative result of 2 independent experiments is shown for E8I in CD4+ T cells. Values are shown as % input. (b) Flow cytometry analysis showing surface CD8α expression on GFP+ wild-type CD4+ T cells 48 hours after transduction with either "empty" MIGR-control or with a Gata3-containing retroviral vector. MS-275 (or DMSO as carrier control) was added 24 hours after transduction. Data are representative of 4 independent experiments. The summary of all experiments is shown at the bottom. For each experiment, the percentage of CD8+ MS275-treated MIGR-transduced GFP+ cells was set to 1 and the relative value of the percentage of CD8+ MS275-treated GATA3-transduced cells was calculated. *P<0.05 (one sample two-tailed t-test). Mean ± SD is shown. (c) Correlation plot of the log2-ratios for microarray transcriptomic (WT/HDAC1-2 cKO) and proteomic data (WT/HDAC1-2 cKO). The pearson's correlation coefficient is 0.4357, and spearman's rank coefficient is 0.4219.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–5 (PDF 2652 kb)
Rights and permissions
About this article
Cite this article
Boucheron, N., Tschismarov, R., Göschl, L. et al. CD4+ T cell lineage integrity is controlled by the histone deacetylases HDAC1 and HDAC2. Nat Immunol 15, 439–448 (2014). https://doi.org/10.1038/ni.2864
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2864
This article is cited by
-
The role of epigenetic modifications in drug resistance and treatment of breast cancer
Cellular & Molecular Biology Letters (2022)
-
Hdac1 and Hdac2 are essential for physiological maturation of a Cx3cr1 expressing subset of T-lymphocytes
BMC Research Notes (2021)
-
Histone deacetylase 2 knockout suppresses immune escape of triple-negative breast cancer cells via downregulating PD-L1 expression
Cell Death & Disease (2021)
-
Conversion of effector CD4+ T cells to a CD8+ MHC II-recognizing lineage
Cellular & Molecular Immunology (2021)
-
Protein acetylation: a novel modus of obesity regulation
Journal of Molecular Medicine (2021)