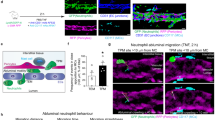
Abstract
Coordinated navigation within tissues is essential for cells of the innate immune system to reach the sites of inflammatory processes, but the signals involved are incompletely understood. Here we demonstrate that NG2+ pericytes controlled the pattern and efficacy of the interstitial migration of leukocytes in vivo. In response to inflammatory mediators, pericytes upregulated expression of the adhesion molecule ICAM-1 and released the chemoattractant MIF. Arteriolar and capillary pericytes attracted and interacted with myeloid leukocytes after extravasating from postcapillary venules, 'instructing' them with pattern-recognition and motility programs. Inhibition of MIF neutralized the migratory cues provided to myeloid leukocytes by NG2+ pericytes. Hence, our results identify a previously unknown role for NG2+ pericytes as an active component of innate immune responses, which supports the immunosurveillance and effector function of extravasated neutrophils and macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chen, G.Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Kono, H. & Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 (2008).
McDonald, B. & Kubes, P. Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J. Mol. Med. 89, 1079–1088 (2011).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Peter, C., Wesselborg, S., Herrmann, M. & Lauber, K. Dangerous attraction: phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis 15, 1007–1028 (2010).
Phillipson, M. & Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 17, 1381–1390 (2011).
Ley, K., Laudanna, C., Cybulsky, M.I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Nourshargh, S., Hordijk, P.L. & Sixt, M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378 (2010).
Woodfin, A. et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 12, 761–769 (2011).
Bajénoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Friedl, P. & Weigelin, B. Interstitial leukocyte migration and immune function. Nat. Immunol. 9, 960–969 (2008).
Armulik, A., Abramsson, A. & Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 97, 512–523 (2005).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Balabanov, R., Beaumont, T. & Dore-Duffy, P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J. Neurosci. Res. 55, 578–587 (1999).
Verbeek, M.M., Westphal, J.R., Ruiter, D.J. & de Waal, R.M. T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J. Immunol. 154, 5876–5884 (1995).
Murfee, W.L., Skalak, T.C. & Peirce, S.M. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation 12, 151–160 (2005).
Proebstl, D. et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med. 209, 1219–1234 (2012).
Lebrin, F. et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat. Med. 16, 420–428 (2010).
Zhu, X., Bergles, D.E. & Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135, 145–157 (2008).
Petrie, R.J., Doyle, A.D. & Yamada, K.M. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 10, 538–549 (2009).
Edelman, D.A., Jiang, Y., Tyburski, J.G., Wilson, R.F. & Steffes, C.P. Lipopolysaccharide activation of pericyte's Toll-like receptor-4 regulates co-culture permeability. Am. J. Surg. 193, 730–735 (2007).
Franchi, L., Eigenbrod, T., Munoz-Planillo, R. & Nunez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 (2009).
Bernhagen, J. et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587–596 (2007).
Santos, L.L. et al. Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis in mice. Arthritis Rheum. 63, 960–970 (2011).
Cheng, Q. et al. Macrophage migration inhibitory factor increases leukocyte-endothelial interactions in human endothelial cells via promotion of expression of adhesion molecules. J. Immunol. 185, 1238–1247 (2010).
Gregory, J.L. et al. Reduced leukocyte-endothelial cell interactions in the inflamed microcirculation of macrophage migration inhibitory factor-deficient mice. Arthritis Rheum. 50, 3023–3034 (2004).
Al-Abed, Y. & VanPatten, S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med. Chem. 3, 45–63 (2011).
Cvetkovic, I. et al. Critical role of macrophage migration inhibitory factor activity in experimental autoimmune diabetes. Endocrinology 146, 2942–2951 (2005).
Leng, L. et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J. Immunol. 186, 527–538 (2010).
Hudson, J.D. et al. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 190, 1375–1382 (1999).
Afonso, P.V. et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091 (2012).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Baumann, R. et al. Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria-dependent death pathway. FASEB J. 17, 2221–2230 (2003).
Luster, A.D., Alon, R. & von Andrian, U.H. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6, 1182–1190 (2005).
Foxman, E.F., Campbell, J.J. & Butcher, E.C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 139, 1349–1360 (1997).
Miller, M.J., Wei, S.H., Parker, I. & Cahalan, M.D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002).
Sumen, C., Mempel, T.R., Mazo, I.B. & von Andrian, U.H. Intravital microscopy: visualizing immunity in context. Immunity 21, 315–329 (2004).
Zachariah, M.A. & Cyster, J.G. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 328, 1129–1135 (2010).
Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 (2010).
Siffrin, V. et al. Differential immune cell dynamics in the CNS cause CD4+ T cell compartmentalization. Brain 132, 1247–1258 (2009).
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 (2007).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods 25, 402–408 (2001).
Faust, N., Varas, F., Kelly, L.M., Heck, S. & Graf, T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96, 719–726 (2000).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Li, J.L. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 (2012).
Ng, L.G. et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest. Dermatol. 131, 2058–2068 (2011).
Kreisel, D. et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl. Acad. Sci. USA 107, 18073–18078 (2010).
Acknowledgements
We thank A. Suhr, N. Blount and A. Vens for technical assistance; T. Graf (Center for Genomic Regulation) for LysM-eGFP mice; and S. Jung (Weizmann Institute of Science) for CX3CR1-GFP mice. Supported by Collaborative Research Center SFB 914 (S.M., A.G.K., K.L. and B.W.), Deutsche Forschungsgemeinschaft Forschergruppe 923 (S.M.) and Framework Programme 7 of the European Union (PRESTIGE; S.M.).
Author information
Authors and Affiliations
Contributions
K.S., A.E., S.H. and S.M. conceived of and designed the experiments; K.S., A.E., S.H., A.G.K. and F.G. established and did two-photon microscopy; R.P. provided the code for the heat-map visualizations; K.S. and S.H. did cell tracking, three-dimensional rendering and heat-map visualizations; K.S., A.E. and M.-L.v.B. planned and did immunohistochemical and whole-mount staining; K.S., A.E., I.H. and B.W. designed and did chemotaxis assays; K.S., A.E., F.G. and K.L. designed and did adhesion and spreading assays; A.E., A.T. and M.L. did RT-PCR, flow cytometry and enzyme-linked immunosorbent assays; K.S., A.E. and S.M. analyzed the data; and K.S., A.E., K.L. and S.M. composed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Tables 1–2 (PDF 6507 kb)
Supplementary Video 1
NG2+ pericytes surrounding CD31+ endothelial cells. The video shows a whole mount staining of a microvascular vessel in the ear of an NG2DsRed mouse. NG2+ pericytes (red) in close contact to CD31+ endothelial cells (green), immunostained by a FITC labelled anti-CD31 antibody. Visualized by 2-photon microscopy. Still image is shown in Supplementary Figure 1d. (MOV 840 kb)
Supplementary Video 2
Interaction of a CX3CR1+ macrophage with a microvascular pericyte during interstitial migration in vivo. The video shows a vessel in the ear skin of an NG2DsRed- CX3CR1eGFP chimera with an NG2+ pericyte (red) around the capillary, stained by i.a. injection of FITC-Dextran (green). The colocalization (yellow, arrow) is zoomed with decreased density in the green and red channel. Inflammation was induced by s.c. injection of TNF and intravital 2-photon microscopy was performed 4 hrs later. A CX3CR1+ macrophage (green, arrowhead) is interacting with a pericyte over a time period of 20 min. Images were acquired at 2 images per minute and the sequence shows a 30 min time period. Still images are shown in Figure 1e. (MOV 1119 kb)
Supplementary Video 3
Interaction of a LysM+ neutrophil with a microvascular pericyte during interstitial migration in vivo. The video shows a polarized LysM+ neutrophil (green) sequentially interacting with NG2+ cells (red) in the ear skin of an NG2DsRed-LysMeGFP chimera. The track of the cell is shown in blue, colocalization is indicated by yellow. Inflammation was induced by s.c. injection of fMLP 2 hrs before the 2-photon imaging was performed. Images were acquired every 45 sec and the sequence shows a 15 min time period. Still image is shown in Figure 1f. (MOV 384 kb)
Supplementary Video 4
LysM+ cells contacting NG2+ cells during undirected interstitial migration. The video shows LysM+ neutrophils (green, cell 1-3) orientating towards NG2+ cells (red) during interstitial migration in the ear skin of an NG2DsRed-LysMeGFP chimera 2 hrs after injection of fMLP. After interaction cell 1 and cell 2 stay in close contact to NG2+ cells and sequentially interact (colocalization in yellow, arrowheads) with them (yellow/cyan track). Cell 3 interacts with a NG2+ cell (green track) and then establishes a long lasting contact (red track). Inflammation was induced by s.c. injection of fMLP 2 hrs before the 2-photon imaging was performed. Images were acquired every 30 sec and the sequence shows a time period of 1 hr. Still image is shown in Figure 2a. (MOV 823 kb)
Supplementary Video 5
CX3CR1+ cells contacting NG2+ cells during random interstitial migration. The video shows the ear skin of an NG2DsRed-CX3CR1eGFP chimera 4 hrs after s.c. injection of TNF. During undirected migration CX3CR1+ macrophages (green) interact with NG2+ cells (red) and stay in close contact to them. Interaction is visualized by colocalization (yellow). The tracks of cells are shown in different colors. Images were acquired at a rate of 2 images per minute and the sequence shows a time period of 50 min. Still image is shown in Figure 2c. (MOV 829 kb)
Supplementary Video 6
Heatmap visualization of LysM+ cell density over time after fMLP or fMLP and ISO-1 injection. Heatmap visualizations are superimposed on the video, color coding the density of interstitial LysM+ cells in the ear skin of NG2DsRed-LysMeGFP chimeras. Black/blue indicating low density, red/white marking areas of high density. Images were acquired at a rate of 2 images per minute by 2-PIVM and the sequence shows a time period of 55 min. Left: The video shows the ear skin 2 hrs after injection of fMLP. Initially, LysM+ cells (green) are diffusely distributed in the interstitial space. Over time, there is a concentration around NG2+ (red) cells. Still images are shown in Figure 4c. Right: The video shows the ear skin 2 hrs after injection of fMLP and 30 min after injection of ISO-1. LysM+ cells are diffusely distributed over the imaging area and there is no orientation towards NG2+ cells. Still images are shown in Figure 4f. (MOV 5772 kb)
Supplementary Video 7
Heatmap visualization of CX3CR1+ cell density over time after injection of TNFα and ISO-1. The video shows the ear skin of an NG2DsRed- CX3CR1eGFP chimera 4 hrs after injection of TNFα and 30 min after injection of ISO-1. A heatmap visualization is superimposed on the video, color coding the density of interstitial CX3CR1+ macrophages. Black/blue indicating low density, red/white marking areas of high density. The distribution of CX3CR1+ cells over the whole imaging area remains stable over the observation period and there is no concentration around NG2+ cells. Images were acquired at a rate of 2 images per minute and the sequence shows a time period of 45 min. Still images are shown in Supplementary Figure 4f. (MOV 702 kb)
Supplementary Video 8
LysM+ cells contacting NG2+ cells during directed interstitial migration induced by laser injury. The video shows the ear skin of an NG2DsRed-LysMeGFP chimera 45 min after induction of a focal necrosis (yellow) by laser treatment. On their way to the laser injury LysM+ neutrophils (green) are contacting NG2+ cells (red) and migrate along them. Interaction is visualized by colocalization (yellow). Images were acquired at a rate of 2 images per minute and the sequence shows a time period of 30 min. Still image is shown in Figure 6a. (MOV 5395 kb)
Supplementary Video 9
CX3CR1+ cells contacting NG2+ cells during interstitial migration induced by laser injury. The video shows the ear skin of an NG2DsRed-CX3CR1eGFP chimera 4 hrs after induction of localized necrosis by laser treatment. During directed migration CX3CR1+ macrophages (green) interact with NG2+ cells (red) and continue their path to the focus of sterile inflammation. Interaction is visualized by colocalization (yellow). The tracks of cells are shown in different colors. Images were acquired at a rate of 2 images per minute and the sequence shows a time period of 75 min. Still image is shown in Supplementary Figure 6g. (MOV 4160 kb)
Supplementary Video 10
LysM+ cells contacting NG2+ cells during interstitial migration induced by laser injury after treatment with ISO-1. The video shows the ear skin of an NG2DsRed-LysMeGFP chimera 45 min after induction of a focal necrosis (yellow) by laser treatment. ISO-1 was injected s.c. 30 min before the laser treatment. On their way to the laser injury LysM+ neutrophils (green) are contacting NG2+ cells (red), but do not follow them. The tracks of cells are shown in different colors. Images were acquired at a rate of 2 images per minute and the sequence shows a time period of 45 min. (MOV 2532 kb)
Supplementary Video 11
CX3CR1+ cells contacting NG2+ cells during interstitial migration induced by laser injury after treatment with ISO-1. The video shows the ear skin of an NG2DsRed-CX3CR1eGFP chimera 4 hrs after induction of localized necrosis by laser treatment. ISO-1 was injected 30 min before the laser injury. CX3CR1+ macrophages (green) shortly interact with NG2+ cells (red) on their way to the focus of sterile inflammation. The tracks of cells are shown in different colors. Images were acquired at a rate of 2 images per minute and the sequence shows a time period of 50 min. Still image is shown in Supplementary Figure 7g. (MOV 1408 kb)
Rights and permissions
About this article
Cite this article
Stark, K., Eckart, A., Haidari, S. et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol 14, 41–51 (2013). https://doi.org/10.1038/ni.2477
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2477
This article is cited by
-
The role of cardiac pericytes in health and disease: therapeutic targets for myocardial infarction
Nature Reviews Cardiology (2024)
-
Pericytes are protective in experimental pneumococcal meningitis through regulating leukocyte infiltration and blood–brain barrier function
Journal of Neuroinflammation (2023)
-
Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging
Nature Nanotechnology (2023)
-
Enhanced pericyte-endothelial interactions through NO-boosted extracellular vesicles drive revascularization in a mouse model of ischemic injury
Nature Communications (2023)
-
Stromal cells in the tumor microenvironment: accomplices of tumor progression?
Cell Death & Disease (2023)