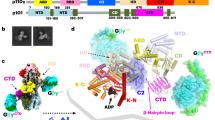
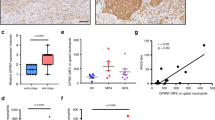
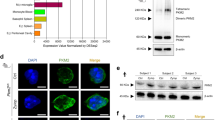
Abstract
To kill invading bacteria, neutrophils must interpret spatial cues, migrate and reach target sites. Although the initiation of chemotactic migration has been extensively studied, little is known about its termination. Here we found that two mitogen-activated protein kinases (MAPKs) had opposing roles in neutrophil trafficking. The extracellular signal–regulated kinase Erk potentiated activity of the G protein–coupled receptor kinase GRK2 and inhibited neutrophil migration, whereas the MAPK p38 acted as a noncanonical GRK that phosphorylated the formyl peptide receptor FPR1 and facilitated neutrophil migration by blocking GRK2 function. Therefore, the dynamic balance between Erk and p38 controlled neutrophil 'stop' and 'go' activity, which ensured that neutrophils reached their final destination as the first line of host defense.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Devreotes, P.N. & Zigmond, S.H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu. Rev. Cell Biol. 4, 649–686 (1988).
Ridley, A.J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Swaney, K.F., Huang, C.H. & Devreotes, P.N. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39, 265–289 (2010).
Janetopoulos, C. & Firtel, R.A. Directional sensing during chemotaxis. FEBS Lett. 582, 2075–2085 (2008).
Xu, J. et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214 (2003).
Srinivasan, S. et al. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 160, 375–385 (2003).
Li, Z. et al. Directional sensing requires G β γ-mediated PAK1 and PIX α-dependent activation of Cdc42. Cell 114, 215–227 (2003).
Xu, J., Wang, F., Van Keymeulen, A., Rentel, M. & Bourne, H.R. Neutrophil microtubules suppress polarity and enhance directional migration. Proc. Natl. Acad. Sci. USA 102, 6884–6889 (2005).
Xu, J. et al. Polarity reveals intrinsic cell chirality. Proc. Natl. Acad. Sci. USA 104, 9296–9300 (2007).
Foxman, E.F., Campbell, J.J. & Butcher, E.C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 139, 1349–1360 (1997).
Lefkowitz, R.J. G protein–coupled receptors, III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J. Biol. Chem. 273, 18677–18680 (1998).
McLeish, K.R., Gierschik, P. & Jakobs, K.H. Desensitization uncouples the formyl peptide receptor–guanine nucleotide-binding protein interaction in HL60 cells. Mol. Pharmacol. 36, 384–390 (1989).
Prossnitz, E.R., Kim, C.M., Benovic, J.L. & Ye, R.D. Phosphorylation of the N-formyl peptide receptor carboxyl terminus by the G protein–coupled receptor kinase, GRK2. J. Biol. Chem. 270, 1130–1137 (1995).
Moore, C.A., Milano, S.K. & Benovic, J.L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 69, 451–482 (2007).
Hsu, M.H., Chiang, S.C., Ye, R.D. & Prossnitz, E.R. Phosphorylation of the N-formyl peptide receptor is required for receptor internalization but not chemotaxis. J. Biol. Chem. 272, 29426–29429 (1997).
Johnson, G.L. & Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 (2002).
Huang, C., Jacobson, K. & Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 117, 4619–4628 (2004).
Nick, J.A. et al. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and fMLP. J. Clin. Invest. 99, 975–986 (1997).
Cara, D.C., Kaur, J., Forster, M., McCafferty, D.M. & Kubes, P. Role of p38 mitogen-activated protein kinase in chemokine-induced emigration and chemotaxis in vivo. J. Immunol. 167, 6552–6558 (2001).
Heit, B., Tavener, S., Raharjo, E. & Kubes, P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159, 91–102 (2002).
Zu, Y.L. et al. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-α or FMLP stimulation. J. Immunol. 160, 1982–1989 (1998).
Hale, K.K., Trollinger, D., Rihanek, M. & Manthey, C.L. Differential expression and activation of p38 mitogen-activated protein kinase α, β, γ, and δ in inflammatory cell lineages. J. Immunol. 162, 4246–4252 (1999).
Nishida, K. et al. p38α mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol. Cell Biol. 24, 10611–10620 (2004).
Sullivan, S.J. & Zigmond, S.H. Chemotactic peptide receptor modulation in polymorphonuclear leukocytes. J. Cell Biol. 85, 703–711 (1980).
Barros, L.F., Young, M., Saklatvala, J. & Baldwin, S.A. Evidence of two mechanisms for the activation of the glucose transporter GLUT1 by anisomycin: p38(MAP kinase) activation and protein synthesis inhibition in mammalian cells. J. Physiol. (Lond.) 504, 517–525 (1997).
Theilade, J., Hansen, J.L., Haunso, S. & Sheikh, S.P. MAP kinase protects G protein–coupled receptor kinase 2 from proteasomal degradation. Biochem. Biophys. Res. Commun. 330, 685–689 (2005).
Theilade, J., Lerche Hansen, J., Haunso, S. & Sheikh, S.P. Extracellular signal–regulated kinases control expression of G protein–coupled receptor kinase 2 (GRK2). FEBS Lett. 518, 195–199 (2002).
Ono, K. & Han, J. The p38 signal transduction pathway: activation and function. Cell. Signal. 12, 1–13 (2000).
Takekawa, M. et al. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 19, 6517–6526 (2000).
Dickinson, R.J. & Keyse, S.M. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J. Cell Sci. 119, 4607–4615 (2006).
Zidar, D.A., Violin, J.D., Whalen, E.J. & Lefkowitz, R.J. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. USA 106, 9649–9654 (2009).
Ye, R.D. et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 (2009).
Butcher, E.C. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67, 1033–1036 (1991).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
Xu, J. et al. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating β2 integrins. Nat. Immunol. 9, 880–886 (2008).
Semmelhack, J.L. & Wang, J.W. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 459, 218–223 (2009).
Acknowledgements
We thank X. Du, B. Gantner (University of Illinois at Chicago), S. Wang (Peking University) and S. Chen (University of Iowa) for discussions, E.R. Prossnitz (University of New Mexico) for the FPR1-ΔST construct and K. Otsu (Osaka University) for permission to use mice with loxP-flanked Mapk14. Supported by the US National Institutes of Health (HL095716 and AI033503), the Chinese Academy of Sciences (KSCX-W-R-66 and KSCX2-YW-R-156), the Natural Science Foundation of China (30630037 and 31070956) and the National Basic Research Program of China (2010CB945301 and 2011CB710900).
Author information
Authors and Affiliations
Contributions
X.L., B.M., Y.L., R.D.Y. and J.X. designed the research; X.L., B.M., H.T., T.Y., B.S. and G.W. did the experiments; X.L., B.M. and J.X. analyzed data; A.B.M., R.D.M. and Y.Z. contributed new reagents and tools; and X.L., B.M., A.B.M., Y.L., R.D.Y. and J.X. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Tables 1–2 (PDF 12697 kb)
Supplementary Video 1
Control HL60 cells migrated in the 100 nM fMLP gradient. (AVI 3844 kb)
Supplementary Video 2
SB203580-treated HL60 cells migrated in the 100 nM fMLP gradient. (AVI 4667 kb)
Supplementary Video 3
PD98059-treated HL60 cells migrated in the 100 nM fMLP gradient. (AVI 4379 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Ma, B., Malik, A. et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol 13, 457–464 (2012). https://doi.org/10.1038/ni.2258
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2258
This article is cited by
-
An Intriguing Structural Modification in Neutrophil Migration Across Blood Vessels to Inflammatory Sites: Progress in the Core Mechanisms
Cell Biochemistry and Biophysics (2024)
-
Dusp6 deficiency attenuates neutrophil-mediated cardiac damage in the acute inflammatory phase of myocardial infarction
Nature Communications (2022)
-
Identification and characterization of neutrophil heterogeneity in sepsis
Critical Care (2021)
-
Long noncoding RNA GSEC promotes neutrophil inflammatory activation by supporting PFKFB3-involved glycolytic metabolism in sepsis
Cell Death & Disease (2021)
-
The principles of directed cell migration
Nature Reviews Molecular Cell Biology (2021)