Abstract
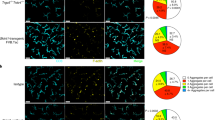
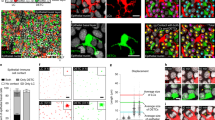
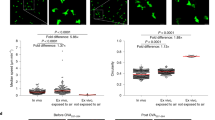
The surveillance of body barriers relies on resident T cells whose repertoires are biased toward particular γδ T cell antigen receptors (TCRs) according to location. These γδ TCRs can recognize ligands that emerge after stress. Through the use of intravital dynamics–immunosignal correlative microscopy, we found that γ-chain variable region 5 (Vγ5) TCRs expressed by epidermal T cells were constitutively clustered and functionally activated in vivo at steady state, forming true immunological synapses that polarized and anchored T cell projections at squamous keratinocyte tight junctions. This synaptogenesis depended on TCR variable domains, the kinase Lck and the integrin αEβ7 but not the γδ lineage or the receptor NKG2D. In response to tissue stress, TCR-proximal signals did not increase substantially but underwent stress mode–dependent relocalization toward the basal epidermis and Langerhans cells. Thus, the γδ TCR orchestrates barrier surveillance proactively, presumably by recognizing tissue ligands expressed in the steady state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
04 April 2012
In the version of this article initially published, the designation for DETCs that lack a Vγ5 TCR is incorrect. The correct designation is 'Vγ5-'. Also, on page 273, right column, second full paragraph, the designation for reporter mice in the first sentence is incorrect. The correct designation is 'IL-2p8–GFP'. The errors have been corrected in the HTML and PDF versions of the article.
References
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
Jameson, J. et al. A role for skin γδ T cells in wound repair. Science 296, 747–749 (2002).
Chen, Y., Chou, K., Fuchs, E., Havran, W.L. & Boismenu, R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc. Natl. Acad. Sci. USA 99, 14338–14343 (2002).
Girardi, M., Lewis, J.M., Filler, R.B., Hayday, A.C. & Tigelaar, R.E. Environmentally responsive and reversible regulation of epidermal barrier function by γδ T cells. J. Invest. Dermatol. 126, 808–814 (2006).
Carding, S.R. & Egan, P.J. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 (2002).
Xiong, N. & Raulet, D.H. Development and selection of γδ T cells. Immunol. Rev. 215, 15–31 (2007).
Bandeira, A. et al. Localization of γδ T cells to the intestinal epithelium is independent of normal microbial colonization. J. Exp. Med. 172, 239–244 (1990).
Groh, V., Steinle, A., Bauer, S. & Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279, 1737–1740 (1998).
Havran, W.L., Chien, Y.H. & Allison, J.P. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science 252, 1430–1432 (1991).
Diefenbach, A., Jamieson, A.M., Liu, S.D., Shastri, N. & Raulet, D.H. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 1, 119–126 (2000).
Strid, J. et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 9, 146–154 (2008).
Hayday, A.C. γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Whang, M.I., Guerra, N. & Raulet, D.H. Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J. Immunol. 182, 4557–4564 (2009).
Havran, W.L. & Allison, J.P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature 335, 443–445 (1988).
Barbee, S.D. et al. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc. Natl. Acad. Sci. USA 108, 3330–3335 (2011).
Boyden, L.M. et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 40, 656–662 (2008).
Jameson, J.M., Cauvi, G., Witherden, D.A. & Havran, W.L. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. 172, 3573–3579 (2004).
Hara, H. et al. Development of dendritic epidermal T cells with a skewed diversity of γδ TCRs in Vδ1-deficient mice. J. Immunol. 165, 3695–3705 (2000).
Mallick-Wood, C.A. et al. Conservation of T cell receptor conformation in epidermal γδ cells with disrupted primary Vγ gene usage. Science 279, 1729–1733 (1998).
Minagawa, M. et al. Homogeneous epithelial γδ T cell repertoire of the skin is shaped through peripheral selection. J. Dermatol. Sci. 25, 150–155 (2001).
Yui, M.A., Sharp, L.L., Havran, W.L. & Rothenberg, E.V. Preferential activation of an IL-2 regulatory sequence transgene in TCR γδ and NKT cells: subset-specific differences in IL-2 regulation. J. Immunol. 172, 4691–4699 (2004).
Hogquist, K.A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992).
Chiba, H., Osanai, M., Murata, M., Kojima, T. & Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta 1778, 588–600 (2008).
Lanier, L.L. DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 227, 150–160 (2009).
Chan, A.C. et al. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14, 2499–2508 (1995).
Kersh, E.N., Shaw, A.S. & Allen, P.M. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science 281, 572–575 (1998).
Schlickum, S. et al. Integrin αE(CD103)β7 influences cellular shape and motility in a ligand-dependent fashion. Blood 112, 619–625 (2008).
Schön, M.P. et al. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J. Immunol. 162, 6641–6649 (1999).
Dustin, M.L., Chakraborty, A.K. & Shaw, A.S. Understanding the structure and function of the immunological synapse. Cold Spring Harb. Perspect. Biol. published online, doi:10.1101/cshperspect.a002311 (15 September 2010).
Freiberg, B.A. et al. Staging and resetting T cell activation in SMACs. Nat. Immunol. 3, 911–917 (2002).
Stinchcombe, J.C., Bossi, G., Booth, S. & Griffiths, G.M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Möbius, W., Herzog, V., Sandhoff, K. & Schwarzmann, G. Intracellular distribution of a biotin-labeled ganglioside, GM1, by immunoelectron microscopy after endocytosis in fibroblasts. J. Histochem. Cytochem. 47, 1005–1014 (1999).
Parton, R.G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42, 155–166 (1994).
Witherden, D.A. et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial γδ T cell activation. Science 329, 1205–1210 (2010).
Saito, T., Yokosuka, T. & Hashimoto-Tane, A. Dynamic regulation of T cell activation and co-stimulation through TCR-microclusters. FEBS Lett. 584, 4865–4871 (2010).
Campi, G., Varma, R. & Dustin, M.L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202, 1031–1036 (2005).
Komano, H. et al. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc. Natl. Acad. Sci. USA 92, 6147–6151 (1995).
Sharp, L.L., Jameson, J.M., Cauvi, G. & Havran, W.L. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 6, 73–79 (2005).
Mohamadzadeh, M. et al. Functional roles for granzymes in murine epidermal γδ T-cell-mediated killing of tumor targets. J. Invest. Dermatol. 107, 738–742 (1996).
Kubo, A., Nagao, K., Yokouchi, M., Sasaki, H. & Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206, 2937–2946 (2009).
Thévenaz, P., Ruttimann, U.E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998).
Acknowledgements
We thank W. Havran (The Scripps Research Institute) for the 7-17 cell line and advice; E. Rothenberg and M. Yui (Caltech) for IL-2p8–GFP mice; M. Nussenzweig (The Rockefeller University) for CD11c-YFP; L. Lanier, J. Beilke and M. Orr (University of California, San Francisco) for tissue samples from DAP10-DAP12–deficient mice; N.R.J. Gascoigne and G. Fu (The Scripps Research Institute), D. Zhou (MD Anderson Cancer Center) and W. Swat (Washington University School of Medicine) for other mutant mice (data not shown); A. Fukunaga for help with tissue processing; D. Nevozhay for help with statistical analysis; and M. Kripke, S. Watowich, C. Zhu, S. Ullrich, K. Newberry and W. Pagel for comments on the manuscript. Supported by the National Institute of Allergy and Infection Diseases (K22-AI065688 to T.Z.), MD Anderson Cancer Center (3-0026138 to T.Z.), the National Cancer Institute (CA016672 to MD Anderson) and institutional startup funds (T.Z.).
Author information
Authors and Affiliations
Contributions
G.C. and T.Z. designed the studies, analyzed and interpreted the results and wrote the manuscript; G.C. obtained most of the data; and V.P. and M.A.Z. assisted and acquired some of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Methods (PDF 6777 kb)
Supplementary Video 1
Apical dendrites are stably anchored. (MOV 992 kb)
Supplementary Video 2
Three-dimensional confocal visualization of DETCs forming apical PALPs in healthy epidermis. (MOV 14131 kb)
Supplementary Video 3
Intravital dynamics-immunosignal correlative microscopy shows that DETC dendrites are anchored in the apical epidermis through PALPs depending on γδTCR. (MOV 670 kb)
Supplementary Video 4
Anchoring of apical dendrites depends on γδ TCR. (MOV 6192 kb)
Supplementary Video 5
Anchoring of apical dendrites depends on Vγ5 TCR. (MOV 1560 kb)
Supplementary Video 6
DETCs remain anchored for days. (MOV 2493 kb)
Supplementary Video 7
DETC dynamics in response to stress stimuli. (MOV 3739 kb)
Supplementary Video 8
Skin inflammation after TLR9 stimulation induces redistribution of TCR signaling. (MOV 10656 kb)
Supplementary Video 9
DETC-Langerhans cell synapse. (MOV 489 kb)
Rights and permissions
About this article
Cite this article
Chodaczek, G., Papanna, V., Zal, M. et al. Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol 13, 272–282 (2012). https://doi.org/10.1038/ni.2240
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2240
This article is cited by
-
Classification and function of γδT cells and its research progress in anti-glioblastoma
Discover Oncology (2023)
-
γδ T cells monitor tissue health
Nature Immunology (2022)
-
Normality sensing licenses local T cells for innate-like tissue surveillance
Nature Immunology (2022)
-
γδ T cells in tissue physiology and surveillance
Nature Reviews Immunology (2021)
-
Skin-resident immune cells actively coordinate their distribution with epidermal cells during homeostasis
Nature Cell Biology (2021)