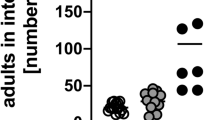
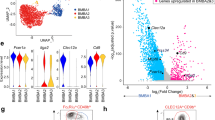
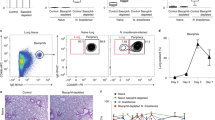
Abstract
Contributions by basophils to allergic and helminth immunity remain incompletely defined. Using sensitive interleukin 4 (Il4) reporter alleles, we demonstrate here that basophil IL-4 production occurs by a CD4+ T cell–dependent process restricted to the peripheral tissues affected. We genetically marked and achieved specific deletion of basophils and found that basophils did not mediate T helper type 2 (TH2) priming in vivo. Two-photon imaging confirmed that basophils did not interact with antigen-specific T cells in lymph nodes but engaged in prolonged serial interactions with T cells in lung tissues. Although targeted deletion of IL-4 and IL-13 in either CD4+ T cells or basophils had a minimal effect on worm clearance, deletion from both lineages demonstrated a nonredundant role for basophil cytokines in primary helminth immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
26 May 2011
In the version of this article initially published, the label ‘DEL-OV’ in the bottom left graph in Figure 5c is incorrect. The correct label is 'DEL-OVA'. The error has been corrected in the HTML and PDF versions of the article.
References
Chan, M.S. The global burden of intestinal nematode infections–fifty years on. Parasitol. Today 13, 438–443 (1997).
Urban, J.F. Jr. Maliszewski, C.R., Madden, K.B., Katona, I.M. & Finkelman, F.D. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J. Immunol. 154, 4675–4684 (1995).
Urban, J.F. Jr. et al. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 (1998).
Voehringer, D., Reese, T.A., Huang, X., Shinkai, K. & Locksley, R.M. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203, 1435–1446 (2006).
Neill, D.R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Price, A.E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 107, 11489–11494 (2010).
Oh, K., Shen, T., Le Gros, G. & Min, B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood 109, 2921–2927 (2007).
Sokol, C.L., Barton, G.M., Farr, A.G. & Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 9, 310–318 (2008).
Yanagihara, Y. et al. Cultured basophils but not cultured mast cells induce human IgE synthesis in B cells after immunologic stimulation. Clin. Exp. Immunol. 111, 136–143 (1998).
Perrigoue, J.G. et al. MHC class II–dependent basophil–CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 10, 697–705 (2009).
Sokol, C.L. et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 10, 713–720 (2009).
Yoshimoto, T. et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide–MHC class II complexes to CD4+ T cells. Nat. Immunol. 10, 706–712 (2009).
Phythian-Adams, A.T. et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J. Exp. Med. 207, 2089–2096 (2010).
Hammad, H. et al. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 207, 2097–2111 (2010).
Ohnmacht, C. et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33, 364–374 (2010).
Tang, H. et al. The T helper type 2 response to cysteine proteases requires dendritic cell–basophil cooperation via ROS-mediated signaling. Nat. Immunol. 11, 608–617 (2010).
Wada, T. et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J. Clin. Invest. 120, 2867–2875 (2010).
Min, B. et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J. Exp. Med. 200, 507–517 (2004).
Voehringer, D., Shinkai, K. & Locksley, R.M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20, 267–277 (2004).
Mohrs, K., Wakil, A.E., Killeen, N., Locksley, R.M. & Mohrs, M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23, 419–429 (2005).
Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R.M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 (2001).
Reinhardt, R.L., Liang, H.E. & Locksley, R.M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10, 385–393 (2009).
van Panhuys, N. et al. Basophils are the major producers of IL-4 during primary helminth infection. J. Immunol. 186, 2719–2728 (2011).
Pearce, E.J. & MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2, 499–511 (2002).
Lantz, C.S. et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392, 90–93 (1998).
Lantz, C.S. et al. IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Lab. Invest. 88, 1134–1142 (2008).
Shen, T. et al. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int. Immunol. 20, 1201–1209 (2008).
Poorafshar, M., Helmby, H., Troye-Blomberg, M. & Hellman, L. MMCP-8, the first lineage-specific differentiation marker for mouse basophils. Elevated numbers of potent IL-4-producing and MMCP-8-positive cells in spleens of malaria-infected mice. Eur. J. Immunol. 30, 2660–2668 (2000).
Gallwitz, M. & Hellman, L. Rapid lineage-specific diversification of the mast cell chymase locus during mammalian evolution. Immunogenetics 58, 641–654 (2006).
Voehringer, D., Liang, H.E. & Locksley, R.M. Homeostasis and effector function of lymphopenia-induced 'memory-like' T cells in constitutively T cell-depleted mice. J. Immunol. 180, 4742–4753 (2008).
Henrickson, S.E. & von Andrian, U.H. Single-cell dynamics of T-cell priming. Curr. Opin. Immunol. 19, 249–258 (2007).
Cyster, J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, e150 (2005).
Voehringer, D., Wu, D., Liang, H.E. & Locksley, R.M. Efficient generation of long-distance conditional alleles using recombineering and a dual selection strategy in replicate plates. BMC Biotechnol. 9, 69 (2009).
Sullivan, B.M. & Locksley, R.M. Basophils: a nonredundant contributor to host immunity. Immunity 30, 12–20 (2009).
Ben-Sasson, S.Z., Le Gros, G., Conrad, D.H., Finkelman, F.D. & Paul, W.E. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc. Natl. Acad. Sci. USA 87, 1421–1425 (1990).
Conrad, D.H., Ben-Sasson, S.Z., Le Gros, G., Finkelman, F.D. & Paul, W.E. Infection with Nippostrongylus brasiliensis or injection of anti-IgD antibodies markedly enhances Fc-receptor-mediated interleukin 4 production by non-B, non-T cells. J. Exp. Med. 171, 1497–1508 (1990).
Le Gros, G. et al. IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J. Immunol. 145, 2500–2506 (1990).
Seder, R.A. et al. Purified FcɛR+ bone marrow and splenic non-B, non-T cells are highly enriched in the capacity to produce IL-4 in response to immobilized IgE, IgG2a, or ionomycin. J. Immunol. 147, 903–909 (1991).
Denzel, A. et al. Basophils enhance immunological memory responses. Nat. Immunol. 9, 733–742 (2008).
Obata, K. et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110, 913–920 (2007).
Ohnmacht, C. & Voehringer, D. Basophil effector function and homeostasis during helminth infection. Blood 113, 2816–2825 (2009).
Kim, S. et al. Cutting edge: Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J. Immunol. 184, 1143–1147 (2010).
Gallwitz, M., Enoksson, M. & Hellman, L. Expression profile of novel members of the rat mast cell protease (rMCP)-2 and (rMCP)-8 families, and functional analyses of mouse mast cell protease (mMCP)-8. Immunogenetics 59, 391–405 (2007).
Mukai, K. et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23, 191–202 (2005).
Finkelman, F.D. et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15, 505–533 (1997).
Finkelman, F.D. et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155 (2004).
Fallon, P.G. et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17, 7–17 (2002).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Veiga-Fernandes, H. et al. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature 446, 547–551 (2007).
Allen, C.D., Okada, T., Tang, H.L. & Cyster, J.G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007).
Lindquist, R.L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Davies, S.J. et al. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science 294, 1358–1361 (2001).
Delmotte, P. & Sanderson, M.J. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 35, 110–117 (2006).
Acknowledgements
We thank K.C. Lim and N. Flores for technical support; E. Thornton and M. Krummel for training in lung-slice preparation; D. Kioussis (Medical Research Council National Institute for Medical Research) for huCD2-DsRed transgenic mice; and J. Cyster (University of California, San Francisco) for mice and two-photon microscope use. Supported by the US National Institutes of Health (AI026918 and AI077439), the Howard Hughes Medical Institute and the Sandler Asthma Basic Research Center at the University of California San Francisco, San Francisco.
Author information
Authors and Affiliations
Contributions
B.M.S., H.-E.L., C.D.C.A. and R.M.L. conceived of the work; H.-E.L. generated Basoph8 reporter mice; B.M.S. designed and did most experiments; C.D.C.A. contributed two-photon imaging data; J.K.B. and B.M.S. analyzed basophils in the small intestine; D.W. and B.M.S. generated data from mice infected with S. mansoni cercariae; L.E.C. analyzed mast cells in the skin in Basoph8 mice; J.K.M. provided S. mansoni cercariae and eggs; and B.M.S., C.D.C.A. and R.M.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–11 (PDF 1074 kb)
Supplementary Video 1
Basophils and antigen-specific CD4+ T cells do not interact in draining lymph nodes after primary immunization with S. mansoni eggs and OVA. A time-lapse sequence of 24 μm z-projection images from an inguinal lymph node explant is shown approximately three days after subcutaneous immunization. Elapsed time is indicated as hh:mm:ss. (MOV 7192 kb)
Supplementary Video 2
Basophils and antigen-specific CD4+ T cells do not interact in draining lymph nodes after primary immunization with papain and OVA. A time-lapse sequence of 32 μm z-projection images from an inguinal lymph node explant is shown approximately 2.25 days after subcutaneous immunization. Elapsed time is indicated as hh:mm:ss. (MOV 5385 kb)
Supplementary Video 3
Antigen-specific CD4+ T cells interact with cognate antigen-specific B cells, but not basophils, in draining lymph nodes after primary immunization. A time-lapse sequence of 33 μm z-projection images from an inguinal lymph node explant is shown approximately 3.7 days after subcutaneous immunization with a mixture of papain and DEL-OVA. Elapsed time is indicated as hh:mm:ss. (MOV 10866 kb)
Supplementary Video 4
Basophils and antigen-specific CD4+ T cells engage in multiple serial interactions in the lung after primary N. brasiliensis infection. A time-lapse sequence of 29 μm z-projection images from a lung slice is shown approximately 7.5 days after N. brasiliensis infection, with intranasal OVA administered on days 1 and 6. Elapsed time is indicated as hh:mm:ss. The video corresponds to the still images shown in Fig. 5a and the characteristics of the serial encounters are quantified in Fig. 5d,e and Supplementary Fig. 8. (MOV 12493 kb)
Supplementary Video 5
Basophils and polyclonal T cells engage in multiple serial interactions in the lung after primary N. brasiliensis infection. A time-lapse sequence of 26 μm z-projection images from a lung slice is shown approximately 7.25 days after N. brasiliensis infection of a Basoph8 mouse carrying a hCD2-dsRed transgene. This transgene is highly expressed in T cells and weakly expressed in subsets of dendritic cells, macrophages, NK cells, and other undefined cell types. In this image sequence, a single green basophil and bright red T cell are shown interacting in the center, with a weakly fluorescent macrophage off to the right side. Elapsed time is indicated as hh:mm:ss. (MOV 4431 kb)
Rights and permissions
About this article
Cite this article
Sullivan, B., Liang, HE., Bando, J. et al. Genetic analysis of basophil function in vivo. Nat Immunol 12, 527–535 (2011). https://doi.org/10.1038/ni.2036
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2036
This article is cited by
-
Single cell transcriptomics clarifies the basophil differentiation trajectory and identifies pre-basophils upstream of mature basophils
Nature Communications (2023)
-
A cardioimmunologist’s toolkit: genetic tools to dissect immune cells in cardiac disease
Nature Reviews Cardiology (2022)
-
Basophils prime group 2 innate lymphoid cells for neuropeptide-mediated inhibition
Nature Immunology (2020)
-
Multicolor two-photon imaging of in vivo cellular pathophysiology upon influenza virus infection using the two-photon IMPRESS
Nature Protocols (2020)
-
Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation
Mucosal Immunology (2020)