Abstract
The heterodimeric cytokine interleukin 27 (IL-27) signals through the IL-27Rα subunit of its receptor, combined with gp130, a common receptor chain used by several cytokines, including IL-6. Notably, the IL-27 subunits p28 (IL-27p28) and EBI3 are not always expressed together, which suggests that they may have unique functions. Here we show that IL-27p28, independently of EBI3, antagonized cytokine signaling through gp130 and IL-6-mediated production of IL-17 and IL-10. Similarly, the ability to generate antibody responses was dependent on the activity of gp130-signaling cytokines. Mice transgenic for expression of IL-27p28 showed a substantial defect in the formation of germinal centers and antibody production. Thus, IL-27p28, as a natural antagonist of gp130-mediated signaling, may be useful as a therapeutic for managing inflammation mediated by cytokines that signal through gp130.
Similar content being viewed by others
Main
Type I cytokines, including interleukin 6 (IL-6; A004204), IL-12, IL-23 and IL-27, are related on the basis of structural motifs, a common four-helix bundle and shared use of receptor subunits1. These cytokines have many biological activities, but their diverse effects on the development of helper T cell subsets have received considerable attention. IL-12 promotes T helper type 1 (TH1) cells; IL-6 and IL-23 are involved in differentiation into IL-17-producing helper T cells (TH17 cells); and IL-27 antagonizes TH1, TH2 and TH17 responses. These ligands signal through membrane-bound receptor complexes that include either gp130 (A001266) or IL-12Rβ1, which activate transcription factor STAT pathways1. Given the role of these cytokines in cell-mediated immunity, it is not unexpected that they are linked to the development of many autoimmune inflammatory conditions2. For example, IL-6 has been linked to control of the recruitment, activation and apoptotic clearance of leukocytes in inflammatory bowel disease, peritonitis, rheumatoid arthritis, Castleman's disease and asthma, which makes IL-6 a viable therapeutic target in these conditions3,4,5.
The receptor subunit gp130 is used by several cytokines, including IL-6, IL-11, IL-27, oncostatin M (OSM), leukemia inhibitory factor (LIF), ciliary neurotrophic factor, cardiotrophin 1 and cardiotrophin-like cytokine (CLC; also known as NNT-1 or BSF-3). Accordingly, these cytokines have similar functions, including induction of acute-phase proteins6, stimulation of hematopoiesis7,8 and promotion of B cell development and antibody production9,10,11,12. However, they also have distinct activities, owing to the use of unique receptor α-chains that pair with gp130 to form functional receptor complexes. For example, the single-subunit cytokine IL-6 binds gp130 in combination with either a membrane-bound or secreted version of the IL-6 receptor α-chain (IL-6Rα; A001265)3,4. IL-27 is a heterodimeric cytokine composed of p28 (IL-27p28), which is a four-helix bundle protein similar to IL-6, and EBI3, which resembles secreted IL-6Rα13. IL-27 uses the unique receptor subunit IL-27Rα (A002911; also known as WSX-1 or TCCR), which pairs with gp130 to initiate signaling13,14.
For the heterodimeric cytokines in the family described above (IL-12, IL-23 and IL-27), models available at present dictate that their secretion is dependent on regulated transcription of the subunits IL-12p35, IL-23p19 and IL-27p28, whereas the subunits p40 and EBI3 are constitutively expressed. For IL-12, this transcriptional regulation may explain why IL-12p40 is produced in excess of IL-12p35, which results in p40 homodimers that can function as IL-12 antagonists15. Although a disulfide bond links IL-12p40 with IL-12p35 or IL-23p19, it is unclear how the subunits of IL-27 interact, which suggests an alternative mechanism of folding and assembly16. Thus, p28 and EBI3 might be secreted independently, allowing association or pairing of each subunit with other proteins. That idea is supported by examples in which EBI3 and p28 are not expressed by the same cells17,18, differences in the transcriptional regulation of each subunit13,19 and evidence that EBI3 and IL-12p35 can associate to form IL-35 (refs. 20,21,22). Nevertheless, on the basis of many bioassays13, no role for IL-27p28 has been reported. However, published work has shown that purified IL-27p28, like heterodimeric IL-27, is able to suppress IL-17 production by CD4+ T cells in vitro23. The basis for this effect is unclear, but it suggests that IL-27p28 could form a complex with EBI3 in culture to form IL-27 or that it could propagate an inhibitory signal on its own. Our studies reported here indicate that IL-27p28, independently of EBI3, blocked the ability of IL-6 to promote TH17 responses and functioned as a natural antagonist of gp130 signaling mediated by IL-6, IL-11 and IL-27. Moreover, transgenic mice overexpressing IL-27p28 showed defective thymus-dependent B cell responses, associated with an inability to form germinal centers (GCs), and a lack of class switching and affinity maturation. Because many cytokines that use gp130 are linked with GC formation and the production of high-affinity antibodies24,25,26,27, these results are consistent with a role for IL-27p28 in blocking these events and suggest that IL-27p28 can act as a natural antagonist of gp130 signaling.
Results
EBI3 is not required for the secretion of IL-27p28
It is unclear whether IL-27p28 can be secreted independently of EBI3 under physiological conditions. Published studies have reported that overexpression of IL-27p28 by established cell lines leads to its secretion independently of EBI3 (refs. 13,28). To determine if IL-27p28 can be secreted by primary cells in the absence of EBI3, we incubated bone marrow–derived macrophages and dendritic cells from wild-type and Ebi3−/− mice with lipopolysaccharide (LPS), interferon-γ (IFN-γ) or a combination of these and assessed secretion of IL-27p28. As reported before29,30, LPS and IFN-γ induced IL-27p28 secretion by wild-type bone marrow–derived macrophages and dendritic cells (Fig. 1a). In addition, these stimuli also resulted in equivalent production of IL-27p28 by Ebi3−/− cells. We obtained similar results with other Toll-like receptor agonists, including CpG (data not shown). Furthermore, infection of wild-type and Ebi3−/− mice induced detectable amounts of IL-27p28 in the serum, with the highest concentrations in the Ebi3−/− mice (Fig. 1b). Together with reports that IL-27p28 and EBI3 can be regulated and produced differently by different cell types13,17,18,19, the finding that IL-27p28 can be secreted in the absence of EBI3 suggests that this subunit may have additional IL-27-independent functions.
(a) Enzyme-linked immunosorbent assay (ELISA) of IL-27p28 production by C57BL/6 wild-type (WT) or Ebi3−/− bone marrow–derived dendritic cells (DC) and macrophages (MΦ) left unstimulated (Unstim) or stimulated for 24 h with IFN-γ, LPS or a combination of LPS and IFN-γ. Data are representative of three independent experiments with similar results. (b) ELISA of IL-27p28 in the serum of wild-type and Ebi3−/− mice isolated before and on days 4 and 8 after infection with Toxoplasma gondii. ND, not detected. Data are representative of three independent experiments with groups of three to four mice (error bars, s.d.). (c,d) Flow cytometry of intracellular IL-17 (c, left) or IL-10 (d, left) and ELISA of the production of IL-17 (c, right) or IL-10 (d, right) in CD4+ T cells isolated from the spleens and lymph nodes of wild-type or Ebi3−/− mice and activated for 4 d with anti-CD3 and anti-CD28 in TH17-polarizing conditions in the presence or absence of IL-27 or IL-27p28, then stimulated for 4 h with PMA and ionomycin in the presence of brefeldin A; ELISAs were done after 72 h of stimulation. Numbers in outlined areas indicate percent IL-17+ cells (c) or IL-10+ cells (d); numbers adjacent to outlined areas indicate the mean fluorescent intensity (MFI) of IL-17+ cells (c) or IL-10+ cells (d). α-, anti-. Data are representative of three independent experiments with similar results with groups of two to three mice (error bars, s.d.).
IL-27p28 is biologically active in the absence of EBI3
Under conditions in which transforming growth factor-β (TGF-β) plus IL-6 is used to induce TH17 development, IL-27 antagonizes IL-17 production, and IL-27p28 alone also has a reproducible inhibitory effect23. However, it is unclear whether IL-27p28 binds to EBI3 present in the cultures to form IL-27, which suppresses TH17 development. When we used splenocytes from Ebi3−/− mice in this assay, IL-27p28 antagonized the production of IL-17 by wild-type and Ebi3−/− CD4+ T cells under TH17-inducing conditions (Fig. 1c). Published studies have shown that TGF-β in combination with IL-6 or IL-27 can induce CD4+ T cells to produce IL-10 (refs. 31,32); however, IL-27p28 alone or in the presence of TGF-β did not support the development of IL-10-producing T cells (data not shown). Furthermore, whereas stimulation of T cells with TGF-β plus IL-6 led to the production of IL-10, this was not affected by the addition of IL-27, but IL-27p28 resulted in a lower capacity of wild-type and Ebi3−/− CD4+ T cells to make IL-10 under these conditions (Fig. 1d). Together these results indicate that the inhibitory effects of IL-27p28 are independent of EBI3 and that it has inhibitory activities distinct from IL-27.
IL-27p28 antagonizes IL-6 and IL-27 STAT signaling
As IL-27p28 can antagonize the ability of IL-6 to promote TH17 differentiation or IL-10 production, we initially hypothesized that IL-27p28 alone could act in a manner analogous to IL-27 and induce STAT signaling to mediate these effects. Because IL-6 and IL-27 activate mainly STAT1 and STAT3, we assessed the ability of IL-27p28 to induce phosphorylation of these proteins in CD4+ T cells. A 15-minute incubation with IL-6 or IL-27 resulted in phosphorylation of STAT1 and STAT3, whereas IL-27p28 alone did not induce STAT phosphorylation (Fig. 2a). This result was consistent at multiple time points examined over a 2-hour period (data not shown). Because IL-6 and IL-27 signal through gp130, an alternative explanation for the inhibitory effects of IL-27p28 was competition with IL-6 for binding to gp130. Therefore, we tested the induction of phosphorylation of STAT1 and STAT3 by IL-6 in the presence of IL-27p28. Incubation of these two proteins together with CD4+ T cells led to much less phosphorylation of STAT1 and STAT3 (Fig. 2a). We obtained a similar result when IL-27p28 was incubated with IL-27 (Fig. 2a). This effect was dose dependent and typically required a 5- to 50-fold excess of IL-27p28 (Supplementary Fig. 1a). Furthermore, phosphorylation of STAT3 by IL-11, a cytokine that uses gp130 but not IL-6Rα or IL-27Rα for signaling, was also lower after the addition of IL-27p28 (Supplementary Fig. 1b). Moreover, when we incubated CD4+ T cells with 'hyper-IL-6', a fusion protein consisting of human IL-6 and secreted IL-6Rα that can signal in trans through gp130 (ref. 33), phosphorylation of STAT1 and STAT3 occurred and this signaling was antagonized by the inclusion of IL-27p28 (Fig. 2b). Notably, the ability of IL-12, which does not signal through gp130, to phosphorylate STAT4 was not blocked by IL-27p28 (Supplementary Fig. 1c).
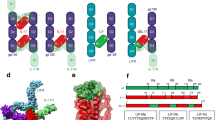
(a,b) Flow cytometry of intracellular phosphorylated STAT1 (p-STAT1) or STAT3 (p-STAT3) in CD4+ T cells purified from wild-type mice and stimulated for 15 min with IL-27p28, IL-6 or IL-27 alone (a) or hyper-IL-6 alone (b) or with IL-6 or IL-27 (a) or hyper-IL-6 (b) plus IL-27p28 preincubated with T cells for 2 h at 37 °C (+ IL-27p28). Numbers in outlined areas indicate percent CD4+ T cells positive for phosphorylated STAT1 or STAT3; numbers adjacent to outlined areas indicate the MFI of phosphorylated STAT1 or STAT3. Data are representative of four independent experiments with similar results. (c) Three-dimensional model of interaction of IL-27p28 with gp130 indicating amino acid residues key to this interaction that differ between IL-27p28 and IL-6. (d) Flow cytometry of intracellular phosphorylated STAT3 in mouse embryonic fibroblasts left unstimulated (gray shaded histograms) or stimulated with OSM or hyper-IL-6 for 15 min at 37 °C (blue lines) or incubated for 2 h at 37 °C with IL-27p28 and then stimulated with OSM or hyper-IL-6 (red lines). Below, change in MFI of phosphorylated STAT3 in mouse embryonic fibroblasts preincubated with IL-27p28 before stimulation with OSM or hyper-IL-6. *P = 0.0059 (unpaired t-test). Data are representative of three individual experiments with similar results (flow cytometry) or five independent experiments (bottom; error bars, s.d.).
IL-27p28 antagonizes the interaction of IL-6 with gp130
The data reported above suggested that IL-27p28 inhibits IL-6 trans signaling by binding to gp130 and not by binding to IL-6Rα, thus limiting the availability of the gp130 receptor subunit for binding to hyper-IL-6. That led us to examine the structural basis for this inhibitory effect. Using the available crystal structure of the human IL-6–gp130 complex as a template, we constructed a three-dimensional model to assess whether IL-27p28 is able to bind gp130 without interacting with EBI3. On the basis of this model, we predicted that similar to IL-6, IL-27p28 would bind the immunoglobulin-like domain of gp130 using amino acid residues located in the AB loop of the protein (Fig. 2c). The amino acid residues predicted to be critical for gp130 binding are Leu81 and Glu85. In this model, Leu81 interacts with Leu3 in gp130 and would result in greater hydrophobicity of IL-27p28 than the serine residue at this position in IL-6. Moreover, Glu85 (a leucine residue in IL-6) would form a salt bridge with the amino terminus of gp130 that is lacking when IL-6 interacts with gp130.
Although the model described above does not resolve whether IL-27p28 must associate with IL-27Rα to antagonize gp130-mediated signaling, the absence of IL-27Rα on T cells did not prevent IL-27p28 from inhibiting STAT phosphorylation in response to IL-6 (data not shown). What this model does indicate is that because of the greater hydrophobicity of IL-27p28 than IL-6, IL-27p28 is able to interact with gp130 in the absence of EBI3 and may antagonize the ability of IL-6, IL-11 and IL-27 to signal through the immunoglobulin-like domain of gp130. The model also suggests that IL-27p28 would not inhibit the effects of other cytokines such as OSM that use the cytokine-binding domain of gp130 (ref. 34). When we stimulated mouse embryonic fibroblasts with hyper-IL-6 or OSM, we observed STAT3 phosphorylation (Fig. 2d), and although the addition of IL-27p28 antagonized this response to hyper-IL-6, IL-27p28 did not inhibit STAT3 phosphorylation by OSM (Fig. 2d). To investigate the ability of IL-27p28 to block the interaction of IL-6 with gp130, we used surface plasmon resonance analysis. The combination of IL-6 and secreted IL-6Rα was able to interact with chip-bound gp130 and induce a binding-curve response (Supplementary Fig. 2). When we introduced IL-27p28 in this system, it resulted in dose-dependent inhibition of the ability of IL-6 and secreted IL-6Rα to interact with gp130. When we used IL-27p28 at a concentration that was tenfold excess relative to IL-6 and secreted IL-6Rα, we observed slightly slower association and faster dissociation as well as an overall lower affinity (lower association constant and higher dissociation constant) of IL-6–secreted IL-6Rα for gp130 (Supplementary Fig. 2). This finding, along with the structural model, suggests that the ability of IL-27p28 to block gp130-mediated signaling is a consequence of a low-affinity interaction.
Analysis of mice transgenic for IL-27p28 expression
To examine the functional role of IL-27p28 in vivo, we cloned the mouse gene encoding IL-27p28 into an expression vector downstream of a regulatory element in which the immunoglobulin intronic heavy-chain enhancer and the proximal promoter for the gene encoding the kinase Lck are juxtaposed to drive expression in B cells and T cells35 (Fig. 3a). We digested plasmid DNA with the restriction enzyme NotI and microinjected the result into oocytes from female B6C3F1 mice fertilized by male C57BL/6J mice. We crossed the founder mice, which aged and reproduced normally, with C57BL/6J mice to form a stable line with transgenic expression of p28 (called 'p28-transgenic' here). We detected basal expression of IL-27p28 by intracellular staining in B cells and T cells from p28-transgenic mice but not in those from wild-type mice (data not shown); this subsequently increased after 48 h of stimulation of the B cell or T cell antigen receptor (Fig. 3b). Those results were also reflected in the amount of IL-27p28 detected in supernatants of the cultures (Supplementary Fig. 3), but we did not detect IL-27p28 in supernatants of resting bone marrow–derived macrophages or dendritic cells from p28-transgenic mice (data not shown). The amount of IL-27p28 in the serum of naive p28-transgenic mice was 10- to 15-fold greater than that detected in the serum of naive wild-type mice (Fig. 3c).
(a) IL-27p28 transgene construct. Functional elements include the juxtaposed Lck proximal promoter (Prom) and immunoglobulin intronic heavy-chain enhancer (Enh); the insertion site for IL-27p28; and a mutated (untranslatable) version of the gene encoding human growth hormone (hGX: filled boxes, exons; open boxes, introns). (b) Flow cytometry of intracellular IL-27p28 in wild-type (blue lines) and p28-transgenic (red lines) CD19+ B cells, CD4+ T cells and CD8+ T cells after stimulation for 48 h with LPS and anti-IgM or activation with anti-CD3 and anti-CD28; cells were incubated for 4 h with brefeldin A before staining. Shaded histogram, fluorescence-minus-phycoerythrin channel. (c) ELISA of IL-27p28 in serum of naive p28-transgenic (p28-TG) mice and their wild-type littermates. (d) Total CD19+B220+ B cells in the spleens of naive p28-transgenic mice and their wild-type littermates, calculated from percentages determined by flow cytometry. (e) Flow cytometry of splenocytes from naive p28-transgenic mice and their wild-type littermates, stained for CD4 and CD8. Numbers adjacent to outlined areas indicate percent CD4+CD8− cells (left) or CD8+CD4− cells (right). (f) Total CD4+ T cells (left) and CD8+ T cells (right) in spleens of the mice in e, calculated from percentages determined by flow cytometry. *P = 0.0024 and **P = 0.0148 (unpaired t-test). (g) Total CD4+CD44hiCD62Llo T cells (left) and CD8+CD44hiCD62Llo T cells (right) in the spleens of p28-transgenic mice and their naive wild-type littermates, calculated from percentages determined by flow cytometry. *P = 0.0062 and **P = 0.0036 (unpaired t-test). Data are representative of two independent experiments (b,c) or three independent experiments with groups of two to four mice (d,f,g) or three to four mice (e; error bars, s.d.).
Assessment of the lymphocyte compartment of p28-transgenic mice showed no difference in the number of mature B cells (Fig. 3d) or any defect in the developmental stages of B-2 cells in the bone marrow or spleen (data not shown). Similarly, the ratio of CD4+ T cells to CD8+ T cells (Fig. 3e) in the spleens of p28-transgenic mice was similar to that of littermate control mice. However, we noted a greater total number of CD4+ and CD8+ T cells in the spleens of p28-transgenic mice (Fig. 3f), associated with more CD4+ T cells and CD8+ T cells with an activated phenotype (CD44hiCD62LloCD69+), than in wild-type mice (Fig. 3g and Supplementary Fig. 4a). Further comparison showed no difference in CD25 expression (Supplementary Fig. 4b), the number of splenic Foxp3+ regulatory T cells (Supplementary Fig. 4c) or production of IL-2, IFN-γ, IL-4, IL-17 and IL-10 after activation of splenocytes for 48 h with antibody to CD3 (anti-CD3) and anti-CD28 (data not shown). Nevertheless, we noted no overt signs of autoimmune disease in p28-transgenic mice as old as 1.5 years of age (data not shown).
To determine if the phenotype of p28-transgenic CD4+ T cells complemented that obtained with recombinant IL-27p28 in vitro, we cultured CD4+ T cells from wild-type and p28-transgenic mice under TH17-inducing conditions and measured IL-17. IL-17 production was limited by transgenic expression of IL-27p28 (Fig. 4a), and p28-transgenic CD4+ T cells produced less IL-10 than wild-type cells did in response to TGF-β plus IL-6 or in response to IL-27 alone (Fig. 4b). As seen with recombinant IL-27p28, overexpression of IL-27p28 by purified CD4+ T cells did not lead to phosphorylation of STAT1 or STAT3 over a 2-hour period of incubation (Fig. 4c and data not shown), whereas preincubation of transgenic CD4+ T cells for 2 h in medium before the addition of IL-6 or IL-27 led to much less phosphorylation of STAT1 and STAT3 (Fig. 4c and Supplementary Fig. 5a). Similarly, after we stimulated p28-transgenic CD4+ T cells with IL-11, they had less phosphorylation of STAT3 than did wild-type CD4+ T cells (Supplementary Fig. 5b). Together these studies indicated that IL-27p28 produced by transgenic cells was able to efficiently antagonize the activity of IL-6, IL-11 and IL-27 (Fig. 1c,d). Given the ability of IL-27p28 to antagonize the development of TH17 cells in vitro, we assessed the capacity of IL-27p28 to inhibit the development of experimental autoimmune encephalomyelitis (EAE), a mouse model of this inflammatory disease of the central nervous system for which IL-17-producing T cells have been suggested to be a cause. We administered a plasmid containing cDNA for IL-27, IL-27p28 or a green fluorescent protein control to C57BL/6J mice 5 d before inducing EAE by immunizing the mice with a peptide of myelin oligodendrocyte glycoprotein amino acids 35–55. Consistent with the in vitro findings, expression of IL-27 greatly inhibited the onset and development of signs of disease in this model, whereas expression of IL-27p28 resulted in a modest delay in the onset and severity of disease relative to that of mice expressing the green fluorescent protein control (Supplementary Fig. 6).
(a,b) Flow cytometry (left) of intracellular IL-17 (a) or IL-10 (b) in CD4+ T cells isolated from the spleens and lymph nodes of wild-type and p28-transgenic mice, activated for 4 d with anti-CD3 and anti-CD28 in nonpolarizing conditions in the presence of TGF-β plus IL-6 (a,b) or IL-27 (b) and stimulated for 4 h with PMA and ionomycin in the presence of brefeldin A. Numbers in outlined areas indicate percent IL-17+ cells (a) or IL-10+ cells (b); numbers adjacent to outlined areas indicate MFI of IL-17+ cells (a) or IL-10+ cells (b). Right, frequency of IL-17+ cells (a) or IL-10+ cells (b) among the CD4+ T cells described above. *P = 0.0002, **P = 0.0009 and ***P = 0.0158 (unpaired t-test). Data are representative of four individual experiments with similar results (error bars, s.d.). (c) Flow cytometry of intracellular phosphorylated STAT1 or STAT3 in CD4+ T cells purified from p28-transgenic mice and then preincubated for 2 h at 37 °C, then left unstimulated or stimulated with IL-6 for 15 min. Numbers in outlined areas indicate percent CD4+ T cells positive for phosphorylated STAT1 or STAT3; numbers adjacent to outlined areas indicate MFI of phosphorylated STAT1 or STAT3. Data are representative of three independent experiments with similar results.
Overexpression of IL-27p28 limits B cell responses
Many cytokines that use gp130 influence the adaptive immune response, including B cell development and antibody production10,11,12. Examination of antibody production showed that naive p28-transgenic mice had a substantially lower total number of cells secreting immunoglobulin M (IgM) and IgG in the spleen and bone marrow than did wild-type mice (Supplementary Fig. 7a,b), which suggested a role for IL-27p28 in the regulation of B cell responses in vivo. We used several experimental systems to evaluate whether overexpression of IL-27p28 could influence antibody production after immunization with thymus-independent and thymus-dependent antigens, which allowed us to delineate many aspects of the B cell response, including extrafollicular IgM production, GC formation and antibody class switching and affinity maturation.
To examine IgM production, we immunized wild-type and p28-transgenic mice intraperitoneally with either the thymus-independent antigen NP-Ficoll (2,4 dinitrophenol (NP) conjugated to Ficoll) in saline or the thymus-dependent antigen NP-CGG (NP conjugated to chicken γ-globulin) in alum and counted antigen-specific antibody-secreting cells by enzyme-linked immunospot assay. There was no substantial difference in the number of NP-specific IgM–secreting cells detected in the spleens of wild-type and p28-transgenic mice (Fig. 5a,b). Thus, transgenic expression of IL-27p28 does not affect the ability of B cells to mount an IgM-specific antibody response to thymus-independent or thymus-dependent antigens.
(a) Enzyme-linked immunospot assay of IgM+ antibody-secreting cells (ASC) able to bind to NP33-BSA in the spleens of naive (untreated (NT)) p28-transgenic mice and their wild-type littermates (control) or 5 d after immunization with NP-Ficoll in saline. (b–d) Enzyme-linked immunospot assay of IgM+ (b) or IgG1+ (c) antibody-secreting cells able to bind NP33-BSA (b,c), or IgG1+ antibody-secreting cells able to bind NP4-BSA (d), in the spleens of naive p28-transgenic mice and their wild-type littermates (control) or 7 d (D7) and 14 d (D14) after immunization with NP-CGG in alum. *P < 0.001 (nonparametric Mann-Whitney U-test). Each symbol represents an individual mouse; small horizontal lines indicate the mean. Data are representative of two independent experiments with groups of two to three mice (a) or two (day 7) or three (day 14) independent experiments with groups of three mice (b–d).
On day 14 after immunization with NP-CGG, wild-type mice were able to effectively generate a low-affinity NP-specific IgG1 response as assesed by binding to NP33-BSA (33 molecules of NP per molecule of bovine serum albumin (BSA)), whereas we detected no antigen-specific IgG1-secreting cells in the spleens of p28-transgenic mice (Fig. 5c). Moreover, assessment of affinity maturation at this time point indicated the presence of IgG1-secreting cells specific for NP4-BSA in the spleens of wild-type mice but not those of p28-transgenic mice (Fig. 5d). When we measured the NP33- and NP4-specific IgG1 responses in bone marrow at day 14 after immunization, we observed neither low-affinity nor high-affinity antigen-specific IgG1–secreting cells in p28-transgenic mice (Supplementary Fig. 8a,b). There were also significantly fewer antigen-specific IgM secreting cells in the bone marrow (P = 0.0003; Supplementary Fig. 8c). At day 56 after immunization, the NP4-specific IgG1 response remained deficient in p28-transgenic mice, in contrast to the response of their littermates (data not shown). One possible explanation for this phenotype is that there was lower survival of antibody-secreting cells in p28-transgenic mice; however, we observed no difference between naive or immunized wild-type and p28-transgenic mice in terms of B cell death in the spleen (data not shown). Furthermore, we detected no NP-specific IgG antibodies in the serum of p28-transgenic mice at day 7 or 14 after immunization (Supplementary Fig. 8d), which suggested that IL-27p28 does not result in lower survival of antibody-secreting cells but instead prevents their development.
To determine the basis for the antibody defect in the models described above, we visualized GCs in the spleen by their binding to peanut agglutinin (PNA); this demonstrated normal splenic architecture in naive wild-type and p28-transgenic mice (Fig. 6a). However, whereas wild-type mice showed typical GC formation and structure at day 14 after immunization with NP-CGG, p28-transgenic mice failed to generate GCs, given the absence of PNA+ B cells inside the follicle (Fig. 6a). As the NP response in C57BL/6J mice is idiotypically restricted36 and is characterized by the use of the λ1 light chain, expression of the λ-chain can be used as a surrogate marker for NP specificity to allow counting of NP+λ+PNA+ GC B cells. Naive wild-type and p28-transgenic mice showed very few if any NP+λ+PNA+ GC B cells in the spleen. There was expansion of this population in wild-type mice at day 14 after immunization with NP-CGG (Fig. 6b,c), but immunized p28-transgenic mice showed minimal population expansion of NP+λ+PNA+ GC B cells (Fig. 6b,c). Furthermore, immunization with another thymus-dependent antigen, keyhole limpet hemocyanin in complete Freund's adjuvent, resulted in the population expansion of GC B cells and class-switched antibody to keyhole limpet hemocyanin in wild-type mice but not in p28-transgenic mice (Supplementary Fig. 9). Together these data indicate that overexpression of IL-27p28 does not affect thymus-independent responses but has a distinct effect on formation of GCs in response to immunization with thymus-dependent antigens.
(a) Sections of spleens from p28-transgenic mice and their wild-type littermates left unimmunized (top) or immunized with NP-CGG in alum and assessed 14 d after immunization (bottom), stained with fluorescein isothiocyanate–conjugated antibody to the T cell antigen receptor β-chain (TCRβ; T cells); PNA conjugated to rhodamine (GC B cells); and Alexa Fluor 647–conjugated anti-IgD (B cell follicles). Original magnification, ×10. (b) Flow cytometry of PNA+ B cells (right) with NP bound to the λ-light chain (left) in spleens of naive p28-transgenic mice and their wild-type littermates (control) or 14 d after immunization with NP-CGG. Numbers in outlined areas indicate percent λ+NP+ B cells (left) or NP+PNA+ GC B cells (right). (c) Total NP+PNA+ B cells in the spleens in b, calculated from percentages determined by flow cytometry. *P = 0.0049 (unpaired t-test). Data are representative of two (a) or three (b,c) independent experiments with groups of three mice (error bars (c), s.d.).
Discussion
The findings reported here indicate a previously unappreciated role for IL-27p28 as a natural antagonist of gp130-mediated signaling in response to IL-6, IL-11 and IL-27 and highlight the increasingly complex biology of the IL-27 subunits. There are many endogenously produced antagonists of cytokine receptors, including IL-12p40 homodimers15, the soluble IL-1 receptor antagonist and IL-18-binding protein, which antagonize the activity of IL-12, IL-1 and IL-18, respectively37. In addition, surface-bound or soluble IL-13Rα2 serves as a decoy receptor for IL-13 binding and functions to regulate TH2 responses38. The data presented here suggest that IL-27p28 can be added to this list of cytokine antagonists.
Although IL-27p28 and EBI3 are known to form IL-27, they can show different patterns of transcription in response to some stimuli13,19. Additionally, the kinetics of their expression differ after activation of monocyte-derived dendritic cells; IL-27p28 expression peaks early after activation, whereas EBI3 expression is sustained and peaks later13. These observations suggest that the individual subunits of IL-27 can have distinct functions. Accordingly, EBI3 binds to IL-12p35 to form IL-35, a cytokine associated with regulatory and effector T cell function20,21. Also, there are reports indicating that IL-23p19 binds to EBI3 (ref. 1) and that IL-27p28 binds the receptor-like protein CLF39; however, whether these complexes form in vivo is unclear. These findings further emphasize the complex combinatorial biology of this family of cytokines and raise questions about whether other subunits in this family have additional biological activities.
Although it is recognized that gp130 is a key receptor subunit for many cytokines, there is still much to learn about the interaction of various ligands with this receptor. Whereas IL-27p28 does not seem able to bind IL-27Rα alone13, the nature of the interaction between IL-27p28 and gp130 remains unclear. A published report has shown that a recombinant protein composed of gp130 and the Fc fragment does not interfere with IL-27 signaling, which suggests that IL-27 must form a complex with IL-27Rα before interacting with gp130 (ref. 40). Similarly, IL-6 has been shown to be unable to solely bind gp130 (refs. 9,41). However, the model described here does indicate that IL-27p28, similar to IL-6, was able to interact with the immunoglobulin-like domain of gp130 and did so without associating with EBI3. The differences between the association of IL-27p28 with gp130 and that of IL-6 with gp130 were due to two differences between IL-27p28 and IL-6 in amino acids in the AB loop that result in greater hydrophobicity of IL-27p28 and thus its affinity for gp130. Notably, it has been shown that alterations in the AB loop of human IL-6 contribute to the ability of mutant forms of IL-6 to antagonize wild-type IL-6 activity42. Furthermore, modeling studies incorporating fluorescence-correlation spectroscopy have proposed that a gp130 homodimer first binds one IL-6–IL-6Rα complex and then engages a second IL-6–IL-6Rα complex at higher ligand concentrations43. One possibility for the inhibitory activity of IL-27p28 is that it may act at the transition binding state, which would indicate that IL-27p28 would block binding of a second IL-6–IL-6Rα complex43. Regardless of which is true, a definitive crystal structure is needed to elucidate how IL-27p28, alone or as part of IL-27, interacts with gp130 to further determine how this subunit antagonizes signaling through gp130.
Published studies have indicated that gp130 signaling cytokines are necessary for the population expansion, differentiation and antibody production of B cells9,10,11,12. Therefore, on the basis of those findings, we investigated the ability of IL-27p28 to antagonize antibody production in response to immunization with thymus-independent and thymus-dependent antigens. Although we observed no defect in IgM production, mice with transgenic overexpression of IL-27p28 had a severe defect in forming GC reactions and IgG class switching in response to immunization with two different thymus-dependent antigens. Together these results suggest that IL-27p28 is a natural antagonist of gp130-mediated GC formation and the development of antigen-specific antibody production in response to thymus-dependent antigens. However, our experiments did not provide a clear indication of which cytokine IL-27p28 blocks in vivo, and there are many potential candidates, including IL-6, CLC and LIF. For example, the original characterization of Il6−/− mice indicated that after infection with vesicular stomatitis virus and vaccinia virus, Il6−/− mice produce five- to tenfold lower virus-neutralizing IgG titers, whereas IgM titers are similar to those of control mice44. Furthermore, Il6−/− mice immunized with NP36-CGG form fewer and smaller GCs and are less able to mount an antigen-specific IgG response45. Also, mice with transgenic expression of a dominant negative version of gp130 show a defect in antigen-specific antibody production for most isotypes other than IgM after immunization with NP conjugated to ovalbumin24. Moreover, mice with transgenic expression of CLC, LIF or IL-6 show B cell hyperplasia and higher concentrations of serum antibodies of most isotypes25,46,47. Additional studies are needed to address these potential targets of IL-27p28 during GC formation.
The dysregulated production of inflammatory cytokines is associated with many autoimmune diseases. Thus, there has been considerable focus on understanding how cytokines interact with their receptor complexes to develop new approaches for managing inflammation. Specifically, identification of the important amino acid residues on the ligand surface that mediate binding is key for the development of mimics with specific biological properties, such as receptor agonists and antagonists. The observations reported here raise the question of whether IL-27p28 can be used as a therapeutic agent for the treatment of inflammatory conditions and malignancies that involve gp130-signaling cytokines. In support of that idea, a study has reported that IL-27p28-expressing myoblasts suppress an allogenic cytotoxic T cell response and prolong graft survival, a result suggested to be due to the ability of IL-27p28 to block IL-27 activity28. Notably, three reports have indicated that single-nucleotide polymorphisms in human IL-27p28 are associated with susceptibility to asthma and inflammatory bowel disease48,49,50, and in one study, lower production of IL-27p28 was associated with greater susceptibility to early-onset inflammatory bowel disease49. A lack of functional IL-27 to serve as an anti-inflammatory mediator in the lung and gut is one likely explanation for such findings. However, an alternative explanation for the greater risk of asthma and inflammatory bowel disease in these patients is that lower production of IL-27p28 leads to enhanced gp130 signaling in these settings. Moreover, there is compelling literature regarding intervention strategies that directly target STAT3 (refs. 51,52) that would be beneficial for the treatment of inflammation-induced gastrointestinal cancers53 and other forms of cancer54. However, STAT3 is a downstream effector of multiple signaling pathways (such as IL-6, IL-10, IL-11, IL-21, IL-23, IL-27, OSM, LIF, EGF, PDGF, HGF and leptin), and its inhibition would probably have broad effects. Therefore, the identification of inhibitors such as IL-27p28 that specifically antagonize IL-6 family–mediated activation of STAT3 would complement a small-molecule approach for the prevention or modification of ongoing disease.
Methods
Mice.
Ebi3−/− mice on a C57BL/6J background were generated by Lexicon Genetics and provided by M.M.E.; Il27ra−/− mice were provided by C.J.M.S.; and wild-type C57BL/6J mice were from Jackson Laboratory. Mice were housed and bred in specific pathogen–free facilities in the Department of Pathobiology at the University of Pennsylvania in accordance with institutional guidelines.
Generation of the p28-transgenic mice.
The open reading frames of the mouse gene encoding IL-27p28 were amplified by PCR, which added FseI and AscI sites. Then, cDNA encoding IL-27p28 (753 base pairs) was cloned into the immunoglobulin intronic heavy-chain enhancer–Lck promoter transgene expression vector, which directs expression mainly to T cells and B cells as described35. Expression cassettes were excised by digestion with NotI and were microinjected into oocytes from female B6C3f1 mice fertilized by male C57BL/6 mice. Microinjection and production of transgenic mice followed published procedures55. Transgenic founders were bred with C57BL/6J mice to generate stable lines of transgenic mice expressing a single allele encoding IL-27p28. The p28-transgenic mice were maintained by being crossed with wild-type C57BL/6J mice (Jackson laboratories), and age- and sex-matched wild-type littermates were used as controls in all experiments. IL-27p28 expression by p28-transgenic mice was confirmed by measurement of IL-27p28 in the serum by IL-27p28-specific ELISA (R&D Systems). These mice were bred and housed according to institutional guidelines.
Production of recombinant mouse IL-27p28 subunit protein.
The mouse gene encoding IL-27p28 (Genbank accession number AY099297) was cloned from activated mouse macrophage cDNA via DNA primer extension. The forward DNA primer 5′-TTCCCAACAGACCCCCTGAGCC-3′ and reverse DNA primer, 5′-TTAGGAATCCCAGGCTGAGCCTG-3′ were used to produce the mature 621–base pair DNA encoding IL-27p28 for expression in a pL promoter system (Invitrogen). The resulting plasmid containing the mature fragment of the gene encoding IL-27p28 was confirmed by nucleotide sequencing and was transfected into competent DH5α Escherichia coli for fermentation and the production of inclusion bodies. Recombinant IL-27p28 inclusion bodies were collected from the bacteria and processed through a refolding platform.
After being folded, proteins were concentrated in a Pellicon cassette concentrator (Millipore) with a molecular size cutoff of 3 kilodaltons. Recombinant proteins were then centrifuged for 45 min at 7,000g (Beckman J2-21 centrifuge) for removal of all insoluble particulates. They were then carefully titrated to a pH of 6.0 and loaded onto a 20 ml ion-exchange column (Pharmacia), followed by elution with a salt gradient from 0 M to 1.0 M sodium chloride. Fractions were separated by 4–20% Tris-glycine SDS-PAGE and pooled. Pooled samples were dialyzed overnight at 4 °C against 10 mM Tris buffer, pH 8.0. The next day, dialyzed samples were loaded onto an 80-μm hydroxylapetite column and a phosphate gradient from 2 mM to 70 mM sodium phosphate, pH 7.5, over 20 column volumes was run for protein elution. Fractions were pooled on the basis of purity and were dialyzed overnight at 4 °C against 10 mM sodium phosphate, pH 7.5. Protein was quantified by the Bradford assay, filtered in a sterile way through a 0.2-μm filter and lyophilized.
Molecular modeling.
The three-dimensional model structure of the p28-gp130 complex was generated with the IL-6–IL-6R–gp130 structure (Protein DataBank accession code, 1p9m) as a template. A fold-recognition algorithm was used to prove that the IL-27p28 sequence was compatible with the architecture of type I cytokines (ProHit package; ProCeryon Biosciences). Sequential alignment obtained by the fold-recognition algorithm was used to build the IL-27p28 model structure. According to this alignment, amino acid residues were exchanged in the template, and insertions and deletions in IL-27p28 were modeled with a database search approach included with the WHATIF molecular modeling software56. The Ribbons software package57 was used for the creation of ribbon diagrams.
T cell differentiation.
CD4+ T cells were isolated from splenocyte samples and lymph nodes depleted of CD8+ and NK1.1+ cells for enrichment for CD4+ T cells by magnetic bead separation (Polysciences). Cells were plated in 96-well round-bottomed plates (Costar) at a density of 5 × 106 cells per ml. Cells were stimulated with anti-CD3 (1 μg/ml; 145-2C11; eBioscience) and anti-CD28 (1 μg/ml; 37.51; eBioscience). For the production of IL-17+ T cells, cultures were supplemented with recombinant mouse IL-6 (10 ng/ml; eBioscience) and human TGF-β1 (1 ng/ml; R&D Systems). In some cases, IL-27 (50 ng/ml; Amgen) or IL-27p28 (100 ng/ml; Shenandoah Biotechnology) was added to the cultures. For the production of IL-10+ T cells, cultures were supplemented with recombinant mouse IL-27 (50 ng/ml; Amgen). Additionally, IFN-γ and IL-4 were neutralized in all cultures with anti-IFN-γ (10 μg/ml; XMG1.2; BioXCell) and anti-IL-4 (10 μg/ml; 11B11; NCI Preclinical repository). CD4+ T cells were supplemented with fresh medium and reagents on day 3 and were collected on day 4. T cells were restimulated with PMA and ionomycin plus brefeldin A (Sigma) before intracellular staining. Cells were stained with the following antibodies: peridinin chlorophyll protein–conjugated anti-CD4 (RM4-5), phycoerythrin-conjugated anti-IL-17 (TC11-18H10) and allophycocyanin-conjugated anti-IL-10 (JES5-16E3; all from BD Biosciences). A FACSCalibur or FACSCanto II (BD Biosciences) was used for flow cytometry, and data were analyzed with FlowJo software (TreeStar).
Intracellular staining for phosphorylated STAT1, STAT3 and STAT4.
T cells were purified from C57BL/6 mice with a mouse T cell enrichment column kit (R&D Systems). Purified T cells (1 × 106) were incubated for 5, 15, 30, 60 and 120 min with IL-6 (10 ng/ml), IL-27 (50 ng/ml) or hyper-IL-6 (20 ng/ml). Additionally, T cells were preincubated for 2 h with IL-27p28 (100 ng/ml) before stimulation with IL-6, IL-27 or hyper-IL-6. Also, purified T cells were cultured for 3 d under TH17-inducing conditions, followed by a 'rest' period of 1 h on ice in serum-free medium before stimulation with IL-11 (10 ng/ml; R&D Systems) or before 2 h of preincubation with IL-27p28. For analysis of the activation of phosphorylated STAT4 in response to IL-12, purified T cells were activated for 2 d with anti-CD3 and anti-CD28 and allowed to 'rest' for 1 h on ice in serum-free medium before stimulation with IL-12 (10 ng/ml; eBioscience) or 2 h of preincubation with IL-27p28. Cells were fixed for 10 min at 37 °C with 2% (wt/vol) paraformaldehyde. After being fixed, cells were made permeable for 30 min on ice with 90% (vol/vol) methanol and were stained with the appropriate antibodies: Alexa Fluor 647–conjugated antibody to STAT1 phosphorylated at Tyr701 (4a; BD Biosciences), STAT3 phosphorylated at Tyr705 (4/P-STAT3; BD Biosciences) or STAT4 phosphorylated at Tyr693 (38/p-Stat4; BD Biosciences) and peridinin chlorophyll protein–conjugated anti-CD4 (RM4-5; BD Biosciences). Samples were run and analyzed as describe above. A FACSCalibur or FACSCanto II (BD Biosciences) was used for flow cytometry, and data were analyzed with FlowJo software
Antibodies and flow cytometry for B cell assays.
Splenocytes and bone marrow cells were collected and stained as described58. Flow cytometry strategies used the following antibodies: Alexa Fluor 700–conjugated anti-CD19 (eBio1D3), Alexa Fluor 750–conjugated anti-B220 (RA3-6B2) and eFluor 450–conjugated anti-IgD (11-26c; all from eBioscience); phycoerythrin-conjugated anti-CXCR4 (2B11/CXCR4), phycoerythrin-indodicarbocyanine–conjugated anti-F4/80 (BM8), anti-CD4 (H129.19), anti-CD8 (53-6.7) and anti-Gr-1 (RB6-8C5), and biotin-conjugated anti-CD3ɛ (145-2C11), anti-F4/80 (BM8) and anti-IgD (11-26; all from BD Pharmingen); fluorescein isothiocyanate–conjugated or phycoerythrin-conjugated antibody to immunoglobulin λ-chain (JC5-1) and phycoerythrin-conjugated antibody to immunoglobulin κ-chain (187.1; all from SouthernBiotech); fluorescein isothiocyanate–conjugated PNA (Sigma); antibody to immunoglobulin κ-chain (187.1; BD) conjugated to the quantum dot (Qdot) Q655 (Q22021MP; Invitrogen); and NP (N1010-100; Biosearch Technologies) conjugated in-house to allophycocyanin (PB20; Prozyme). Live cells were assessed by preincubation with AmCyan LIVE/DEAD Fixable Dead Cell Stain (Invitrogen). Cells were fixed and made permeable with Solution A and Solution B (Caltag). An LSR II (BD) was used for flow cytometry, followed by analysis with FlowJo software (TreeStar).
Enzyme-linked immunospot assay.
Multiscreen HTS plates (Millipore) were coated with antibody to immunoglobulin heavy and light chains, NP33-BSA or NP4-BSA in sodium bicarbonate buffer. Plates were blocked with 2% (wt/vol) BSA. Cells were incubated undisturbed in the plate for 4–6 h at 37 °C. Biotin-conjugated antibody to immunoglobulin λ-chain and/or κ-chain (Southern Biotech) was added, followed by streptavidin–alkaline phosphatase (Sigma). Spots were detected with BCIP-NBT (5-bromo-4-chloro-3-indolylphosphate–nitro blue tetrazolium; Sigma), then were scanned and counted with an ImmunoSpot Analyzer (Cellular Technology).
Immunization with NP-Ficoll and NP-CGG.
NP coupled to BSA at a high or low molar-substitution ratio (NP33-BSA and NP4-BSA) was used as the solid-phase adsorbent in ELISA for quantification of high-affinity versus low-affinity NP-specific IgG serum antibody. The p28-transgenic mice and their wild-type littermates were immunized intraperitoneally with 50–100 μg NP16-CGG in alum or 100 μg NP50-Ficoll in saline as described59. Mice were killed on day 5 after immunization with NP-Ficoll; mice immunized with NP-CGG were examined on days 7, 14 and 56 after immunization. All NP reagents were from Biosearch Technologies.
Immunohistochemistry.
Spleens were immersed in optimum cutting temperature compound (Tissue Tek) and flash-frozen with 2-methylbutane and liquid nitrogen, then stored at −20 °C. Later, sections 7 μm in thickness were sliced with a cryostat (Zeiss HM505E), fixed with cold acetone and stored at −20 °C. Before being stained, sections were rehydrated in PBS and were incubated with PBS containing 10% (vol/vol) goat serum. Sections were stained with PNA conjugated to rhodamine (Vector Labs), Alexa Fluor 647–conjugated anti-IgD (11-26; eBioscience) and fluorescein isothiocyanate–conjugated anti-CD3-FITC (H57-507; eBioscience). Sections were mounted with Biomedia Gel/Mount (Electron Microscopy Sciences) and were visualized on an LSM-510 Meta confocal microscope (Zeiss).
Statistics.
An unpaired Student's t-test and nonparametric Mann-Whitney U-test were used to determine the significance of differences; P values of less than 0.05 were considered significant.
Accession codes.
UCSD-Nature Signaling Gateway (http://www.signaling-gateway.org): A004204, A001266, A001265 and A002911.
Accession codes
Change history
21 January 2011
In the version of this article initially published, the author name M. Merle Elloso and the associated affiliation were incorrect. The correct affiliation is Centocor Research and Development, Inc. The error has been corrected in the HTML and PDF versions of the article.
References
Kastelein, R.A., Hunter, C.A. & Cua, D.J. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25, 221–242 (2007).
Damsker, J.M., Hansen, A.M. & Caspi, R.R. Th1 and Th17 cells: adversaries and collaborators. Ann. NY Acad. Sci. 1183, 211–221 (2010).
Jones, S.A. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175, 3463–3468 (2005).
Jones, S.A., Richards, P.J., Scheller, J. & Rose-John, S. IL-6 transsignaling: the in vivo consequences. J. Interferon Cytokine Res. 25, 241–253 (2005).
Ding, C., Cicuttini, F., Li, J. & Jones, G. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin. Investig. Drugs 18, 1457–1466 (2009).
Benigni, F. et al. Six different cytokines that share GP130 as a receptor subunit, induce serum amyloid A and potentiate the induction of interleukin-6 and the activation of the hypothalamus-pituitary-adrenal axis by interleukin-1. Blood 87, 1851–1854 (1996).
Hangoc, G. et al. In vivo effects of recombinant interleukin-11 on myelopoiesis in mice. Blood 81, 965–972 (1993).
Metcalf, D., Nicola, N.A. & Gearing, D.P. Effects of injected leukemia inhibitory factor on hematopoietic and other tissues in mice. Blood 76, 50–56 (1990).
Muraguchi, A. et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J. Exp. Med. 167, 332–344 (1988).
Muraguchi, A. et al. T cell-replacing factor- (TRF) induced IgG secretion in a human B blastoid cell line and demonstration of acceptors for TRF. J. Immunol. 127, 412–416 (1981).
Senaldi, G. et al. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc. Natl. Acad. Sci. USA 96, 11458–11463 (1999).
Larousserie, F. et al. Differential effects of IL-27 on human B cell subsets. J. Immunol. 176, 5890–5897 (2006).
Pflanz, S. et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16, 779–790 (2002).
Pflanz, S. et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231 (2004).
Heinzel, F.P., Hujer, A.M., Ahmed, F.N. & Rerko, R.M. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 158, 4381–4388 (1997).
Batten, M. & Ghilardi, N. The biology and therapeutic potential of interleukin 27. J. Mol. Med. 85, 661–672 (2007).
Devergne, O. et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J. Virol. 70, 1143–1153 (1996).
Maaser, C., Egan, L.J., Birkenbach, M.P., Eckmann, L. & Kagnoff, M.F. Expression of Epstein-Barr virus-induced gene 3 and other interleukin-12-related molecules by human intestinal epithelium. Immunology 112, 437–445 (2004).
Sonobe, Y. et al. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 1040, 202–207 (2005).
Collison, L.W. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007).
Niedbala, W. et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 37, 3021–3029 (2007).
Devergne, O., Birkenbach, M. & Kieff, E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc. Natl. Acad. Sci. USA 94, 12041–12046 (1997).
Stumhofer, J.S. et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7, 937–945 (2006).
Kumanogoh, A. et al. Impairment of antigen-specific antibody production in transgenic mice expressing a dominant-negative form of gp130. Proc. Natl. Acad. Sci. USA 94, 2478–2482 (1997).
Senaldi, G. et al. Regulatory effects of novel neurotrophin-1/B cell-stimulating factor-3 (cardiotrophin-like cytokine) on B cell function. J. Immunol. 168, 5690–5698 (2002).
Takatsuki, F. et al. Human recombinant IL-6/B cell stimulatory factor 2 augments murine antigen-specific antibody responses in vitro and in vivo. J. Immunol. 141, 3072–3077 (1988).
Nurieva, R.I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Shimozato, O. et al. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology 128, e816–e825 (2009).
Liu, J., Guan, X. & Ma, X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-γ-mediated pathways. J. Exp. Med. 204, 141–152 (2007).
Molle, C. et al. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J. Immunol. 178, 7607–7615 (2007).
Stumhofer, J.S. et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 (2007).
McGeachy, M.J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Fischer, M. et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat. Biotechnol. 15, 142–145 (1997).
Timmermann, A. et al. Different epitopes are required for gp130 activation by interleukin-6, oncostatin M and leukemia inhibitory factor. FEBS Lett. 468, 120–124 (2000).
Iritani, B.M., Forbush, K.A., Farrar, M.A. & Perlmutter, R.M. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 16, 7019–7031 (1997).
Jack, R.S., Imanishi-Kari, T. & Rajewsky, K. Idiotypic analysis of the response of C57BL/6 mice to the (4-hydroxy-3-nitrophenyl)acetyl group. Eur. J. Immunol. 7, 559–565 (1977).
Arend, W.P., Palmer, G. & Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 (2008).
Mentink-Kane, M.M. et al. IL-13 receptor α2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc. Natl. Acad. Sci. USA 101, 586–590 (2004).
Crabe, S. et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J. Immunol. 183, 7692–7702 (2009).
Scheller, J., Schuster, B., Holscher, C., Yoshimoto, T. & Rose-John, S. No inhibition of IL-27 signaling by soluble gp130. Biochem. Biophys. Res. Commun. 326, 724–728 (2005).
Hibi, M. et al. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63, 1149–1157 (1990).
Brakenhoff, J.P., Bos, H.K., Grotzinger, J., Rose-John, S. & Aarden, L.A. Identification of residues in the putative 5th helical region of human interleukin-6, important for activation of the IL-6 signal transducer, gp130. FEBS Lett. 395, 235–240 (1996).
Schroers, A. et al. Dynamics of the gp130 cytokine complex: a model for assembly on the cellular membrane. Protein Sci. 14, 783–790 (2005).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Wu, Y. et al. IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation. Int. Immunol. 21, 745–756 (2009).
Fattori, E. et al. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood 83, 2570–2579 (1994).
Shen, M.M. et al. Expression of LIF in transgenic mice results in altered thymic epithelium and apparent interconversion of thymic and lymph node morphologies. EMBO J. 13, 1375–1385 (1994).
Chae, S.C. et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J. Hum. Genet. 52, 355–361 (2007).
Imielinski, M. et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 41, 1335–1340 (2009).
Li, C.S. et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J. Gastroenterol. Hepatol. 24, 1692–1696 (2009).
O'Shea, J. J. Targeting the Jak/STAT pathway for immunosuppression. Ann. Rheum. Dis. 63, ii67–ii71 (2004).
O'Shea, J.J., Pesu, M., Borie, D.C. & Changelian, P.S. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat. Rev. Drug Discov. 3, 555–564 (2004).
Howlett, M., Menheniott, T.R., Judd, L.M. & Giraud, A.S. Cytokine signalling via gp130 in gastric cancer. Biochim. Biophys. Acta 1793, 1623–1633 (2009).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Hogan, B., Beddington, R. Costantini, F. & Lacy, E. Manipulating the Mouse Embryo 2nd edn. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA, 1994).
Vriend, G. WHAT IF: a molecular modeling and drug design program. J. Mol. Graph. 8, 52–56 (1990).
Carson, M. Ribbons. Methods Enzymol. 277, 493–505 (1997).
Allman, D.M., Ferguson, S.E., Lentz, V.M. & Cancro, M.P. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J. Immunol. 151, 4431–4444 (1993).
Scholz, J.L. et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc. Natl. Acad. Sci. USA 105, 15517–15522 (2008).
Acknowledgements
We thank D. Gorman for generating the IL-27 and IL-27p28 minicircles. Supported by the US National Institutes for Health (AI-042334 to C.A.H.; AI-054488 to M.P.C.; 1-T32-AI-055428 to J.S.S. and W.J.Q. III; and 2-T32-AI-007532-11 to E.D.T.), the Abramson Cancer Center (Center for Digestive Diseases), the state of Pennsylvania, the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB415, B5 to S.R-J., and SFB415, B7 to J.G. and B.S.), the Cluster of Excellence 'Inflammation at Interfaces' and the Marie Lowe Cancer Center of the University of Pennsylvania (C.A.H.).
Author information
Authors and Affiliations
Contributions
J.S.S. and C.A.H. contributed to all studies and wrote the manuscript; E.D.T., W.J.Q. III, N.H., M.P.C. and S.D.L. were involved in analyzing p28-transgenic mice; R.G. contributed to studies of GC formation; C.J.M.S. contributed to the studies of Il27ra−/− mice; M.M.E. contributed to studies with Ebi3−/− mice; A.C.O. contributed to studies of intracellular staining for IL-27p28; B.S., S.R.-J. and J.G. did the p28-gp130 modeling and contributed to its analysis; C.A.F. and S.A.J. did the biacore assays and contributed to their analysis; M.L.J. provided the recombinant IL-27p28 protein; and Y.C. and D.J.C. did hydrodynamics-based transfection experiments with minicircle DNA and contributed to their analysis.
Corresponding author
Ethics declarations
Competing interests
C.A.H. and J.S.S. have a patent application on the use of p28 to limit gp130 signaling. N.H. and S.D.L. are employees of ZymoGenetics; Y.C. and D.J.C. are employees of DNAX Discovery Research; M.L.J. is an employee of Shenandoah Biotechnology; C.J.M.S. is an employee of Amgen; and M.M.E. is an employee of Centocor Research and Development.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1207 kb)
Rights and permissions
About this article
Cite this article
Stumhofer, J., Tait, E., III, W. et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol 11, 1119–1126 (2010). https://doi.org/10.1038/ni.1957
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1957
This article is cited by
-
Cell type specific IL-27p28 (IL-30) deletion in mice uncovers an unexpected regulatory function of IL-30 in autoimmune inflammation
Scientific Reports (2023)
-
Systemic IL-27 administration prevents abscess formation and osteolysis via local neutrophil recruitment and activation
Bone Research (2022)
-
Dendritic cell-derived IL-27 p28 regulates T cell program in pathogenicity and alleviates acute graft-versus-host disease
Signal Transduction and Targeted Therapy (2022)
-
IL-30† (IL-27A): a familiar stranger in immunity, inflammation, and cancer
Experimental & Molecular Medicine (2021)
-
The clinical significance of IL-6 s and IL-27 s in Bronchoalveolar lavage fluids from children with mycoplasma pneumoniae pneumonia
BMC Infectious Diseases (2020)