Abstract
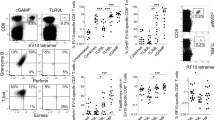
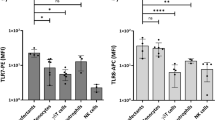
Cross-priming allows dendritic cells (DCs) to induce cytotoxic T cell (CTL) responses to extracellular antigens. DCs require cognate 'licensing' for cross-priming, classically by helper T cells. Here we demonstrate an alternative mechanism for cognate licensing by natural killer T (NKT) cells recognizing microbial or synthetic glycolipid antigens. Such licensing caused cross-priming CD8α+ DCs to produce the chemokine CCL17, which attracted naive CTLs expressing the chemokine receptor CCR4. In contrast, DCs licensed by helper T cells recruited CTLs using CCR5 ligands. Thus, depending on the type of antigen they encounter, DCs can be licensed for cross-priming by NKT cells or helper T cells and use at least two independent chemokine pathways to attract naive CTLs. Because these chemokines acted synergistically, this can potentially be exploited to improve vaccinations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Carbone, F.R., Kurts, C., Bennett, S.R., Miller, J.F. & Heath, W.R. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol. Today 19, 368–373 (1998).
den Haan, J.M., Lehar, S.M. & Bevan, M.J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000).
Shortman, K. & Naik, S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7, 19–30 (2007).
Kurts, C., Kosaka, H., Carbone, F.R., Miller, J.F. & Heath, W.R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J. Exp. Med. 186, 239–245 (1997).
Lukacs-Kornek, V. et al. The kidney-renal lymph node-system contributes to cross-tolerance against innocuous circulating antigen. J. Immunol. 180, 706–715 (2008).
Bennett, S.R., Carbone, F.R., Karamalis, F., Miller, J.F. & Heath, W.R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186, 65–70 (1997).
Bevan, M.J. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 4, 595–602 (2004).
Smith, C.M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol. 5, 1143–1148 (2004).
Janssen, E.M. et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005).
Lutz, M.B. & Kurts, C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur. J. Immunol. 39, 2325–2330 (2009).
Bromley, S.K., Mempel, T.R. & Luster, A.D. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9, 970–980 (2008).
Castellino, F. et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440, 890–895 (2006).
Heymann, F. et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Invest. 119, 1286–1297 (2009).
Hugues, S. et al. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat. Immunol. 8, 921–930 (2007).
Fujii, S., Shimizu, K., Kronenberg, M. & Steinman, R.M. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat. Immunol. 3, 867–874 (2002).
Stober, D., Jomantaite, I., Schirmbeck, R. & Reimann, J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J. Immunol. 170, 2540–2548 (2003).
Fujii, S., Liu, K., Smith, C., Bonito, A.J. & Steinman, R.M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199, 1607–1618 (2004).
Godfrey, D.I. & Kronenberg, M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
Van Kaer, L. NKT cells: T lymphocytes with innate effector functions. Curr. Opin. Immunol. 19, 354–364 (2007).
Tupin, E., Kinjo, Y. & Kronenberg, M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417 (2007).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Kinjo, Y. et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7, 978–986 (2006).
Miyamoto, K., Miyake, S. & Yamamura, T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534 (2001).
Florence, W.C. et al. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d-restricted ligands. EMBO J. 28, 3579–3590 (2009).
Scott-Browne, J.P. et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat. Immunol. 8, 1105–1113 (2007).
Yoshimoto, T. & Paul, W.E. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179, 1285–1295 (1994).
Kitamura, H. et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189, 1121–1128 (1999).
Fujii, S., Shimizu, K., Smith, C., Bonifaz, L. & Steinman, R.M. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198, 267–279 (2003).
Hermans, I.F. et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 171, 5140–5147 (2003).
Kim, C.H., Johnston, B. & Butcher, E.C. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+Vβ11+ NKT cell subsets with distinct cytokine-producing capacity. Blood 100, 11–16 (2002).
Thomas, S.Y. et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J. Immunol. 171, 2571–2580 (2003).
Meyer, E.H. et al. iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity. J. Immunol. 179, 4661–4671 (2007).
Andrew, D.P. et al. C–C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J. Immunol. 166, 103–111 (2001).
Freeman, C.M. et al. CCR4 participation in Th type 1 (mycobacterial) and Th type 2 (schistosomal) anamnestic pulmonary granulomatous responses. J. Immunol. 177, 4149–4158 (2006).
Sallusto, F., Lenig, D., Mackay, C.R. & Lanzavecchia, A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187, 875–883 (1998).
Campbell, J.J. et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400, 776–780 (1999).
Kawasaki, S. et al. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 166, 2055–2062 (2001).
Alferink, J. et al. Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J. Exp. Med. 197, 585–599 (2003).
Horikawa, T. et al. IFN-γ-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int. Immunol. 14, 767–773 (2002).
Campbell, J.J., O'Connell, D.J. & Wurbel, M.A. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J. Immunol. 178, 3358–3362 (2007).
Katou, F. et al. Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissue. Am. J. Pathol. 158, 1263–1270 (2001).
Lieberam, I. & Forster, I. The murine β-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur. J. Immunol. 29, 2684–2694 (1999).
Gonzalo, J.A. et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163, 403–411 (1999).
de Lavareille, A. et al. Clonal Th2 cells associated with chronic hypereosinophilia: TARC-induced CCR4 down-regulation in vivo. Eur. J. Immunol. 31, 1037–1046 (2001).
Kondo, T. & Takiguchi, M. Human memory CCR4+CD8+ T cell subset has the ability to produce multiple cytokines. Int. Immunol. 21, 523–532 (2009).
Kaech, S.M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
van Stipdonk, M.J. et al. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 4, 361–365 (2003).
Long, X. et al. Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat. Chem. Biol. 3, 559–564 (2007).
Du, W., Kulkarni, S.S. & Gervay-Hague, J. Efficient, one-pot syntheses of biologically active α-linked glycolipids. Chem. Commun. (Camb.) 23, 2336–2338 (2007).
Acknowledgements
We thank W. Keßler (University of Greifswald) for CCR4-deficient mice; F. Tacke (University of Aachen) for CD1d- and MHC class II–deficient mice; R. Goldszmid (National Institute of Allergy and Infectious Diseases, National Institutes of Health) for CD8-deficient mice; J. Alferink (University of Bonn) for CCL17-eGFP knock-in mice; A. Peters for technical assistance; C. Coch and Rolf Fimmers for advice on statistics; and the Central Animal Facilities and the Flow Cytometry Core Facility at the Institutes of Molecular Medicine and Experimental Immunology for support. Supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 704 grants A1 (I.F.), A2 (C.K.), A5 (P.A.K.) and A8 (W.K.), and Klinische Forschergruppe 228 grants P1 (U.P.) and P5 (C.K.)), the Australian National Health and Medical Research Council (D.I.G. and J.R.) and the Australian Research Council (J.R.).
Author information
Authors and Affiliations
Contributions
V.S. and V.L-K. designed and did most experiments, analyzed and interpreted data and contributed to the writing of the manuscript; C.A.T., T.Q. and K.H. designed, did and analyzed individual experiments; U.P., J.R., P.P., J.C., D.I.G., P.B.S., P.A.K., W.K. and I.F. contributed tools, discussed and interpreted results and edited the manuscript; and C.K. conceived the project, designed and interpreted experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16 (PDF 3572 kb)
Supplementary Movie 1
Short DC contact duration when CTL had not been exposed to α-GC. (MOV 962 kb)
Supplementary Movie 2
Long DC contact duration when CTL had been exposed to α-GC. (MOV 833 kb)
Supplementary Movie 3
Impaired migration of CCR4-competent CTL towards DCs that cannot produce CCL17 (MOV 1035 kb)
Supplementary Movie 4
Impaired migration of CCR4-deficient CTL towards CCL17-producing DCs. (MOV 1161 kb)
Supplementary Movie 5
Direct comparison of migration of CCR4-competent and –deficient CTL towards CCL17-producing DCs. (MOV 1219 kb)
Rights and permissions
About this article
Cite this article
Semmling, V., Lukacs-Kornek, V., Thaiss, C. et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell–licensed DCs. Nat Immunol 11, 313–320 (2010). https://doi.org/10.1038/ni.1848
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1848
This article is cited by
-
Multi-feature-Based Robust Cell Tracking
Annals of Biomedical Engineering (2023)
-
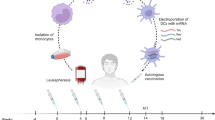
A randomised controlled trial of long NY-ESO-1 peptide-pulsed autologous dendritic cells with or without alpha-galactosylceramide in high-risk melanoma
Cancer Immunology, Immunotherapy (2023)
-
A molecular signature of lung-resident CD8+ T cells elicited by subunit vaccination
Scientific Reports (2022)
-
Heterotypic immunity against vaccinia virus in an HLA-B*07:02 transgenic mousepox infection model
Scientific Reports (2020)
-
Dendritic cell subsets in T cell programming: location dictates function
Nature Reviews Immunology (2019)