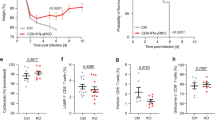
Abstract
We used a sensitive method based on tetramers of peptide and major histocompatibility complex II (pMHCII) to determine whether CD4+ memory T cells resemble the T helper type 1 (TH1) and interleukin 17 (IL-17)-producing T helper (TH17) subsets described in vitro. Intravenous or intranasal infection with Listeria monocytogenes induced pMHCII-specific CD4+ naive T cells to proliferate and produce effector cells, about 10% of which resembled TH1 or TH17 cells, respectively. TH1 cells were also present among the memory cells that survived 3 months after infection, whereas TH17 cells disappeared. The short lifespan of TH17 cells was associated with small amounts of the antiapoptotic protein Bcl-2, the IL-15 receptor and the receptor CD27, and little homeostatic proliferation. These results suggest that TH1 cells induced by intravenous infection are more efficient at entering the memory pool than are TH17 cells induced by intranasal infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jenkins, M.K. et al. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19, 23–45 (2001).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Reinhardt, R.L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M.K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Seder, R.A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4, 835–842 (2003).
Zhou, L., Chong, M.M. & Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Reiner, S.L. & Locksley, R.M. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13, 151–177 (1995).
Swain, S.L., Hu, H. & Huston, G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999).
Lohning, M. et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J. Exp. Med. 205, 53–61 (2008).
Li, J., Huston, G. & Swain, S.L. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198, 1807–1815 (2003).
Hataye, J., Moon, J.J., Khoruts, A., Reilly, C. & Jenkins, M.K. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312, 114–116 (2006).
Marzo, A.L. et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 6, 793–799 (2005).
Badovinac, V.P., Haring, J.S. & Harty, J.T. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8+ T cell response to infection. Immunity 26, 827–841 (2007).
Rees, W. et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4+ T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. USA 96, 9781–9786 (1999).
Moon, J.J. et al. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213 (2007).
Ertelt, J.M. et al. Selective priming and expansion of antigen-specific Foxp3− CD4+ T cells during Listeria monocytogenes infection. J. Immunol. 182, 3032–3038 (2009).
Muller, W.J. et al. Recombinant Listeria monocytogenes expressing an immunodominant peptide fails to protect after intravaginal challenge with herpes simplex virus-2. Arch. Virol. 153, 1165–1169 (2008).
Portnoy, D.A., Auerbuch, V. & Glomski, I.J. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158, 409–414 (2002).
Moon, J.J. et al. Tracking epitope-specific T cells. Nat. Protoc. 4, 565–581 (2009).
Kaech, S.M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002).
Khoruts, A., Mondino, A., Pape, K.A., Reiner, S.L. & Jenkins, M.K. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J. Exp. Med. 187, 225–236 (1998).
Pape, K.A., Merica, R., Mondino, A., Khoruts, A. & Jenkins, M.K. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 160, 4719–4729 (1998).
Szabo, S.J. et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 (2000).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Woolard, M.D., Hensley, L.L., Kawula, T.H. & Frelinger, J.A. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of γ interferon-positive T cells. Infect. Immun. 76, 2651–2659 (2008).
Zhang, Z., Clarke, T.B. & Weiser, J.N. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119, 1899–1909 (2009).
Park, H.S. et al. Primary induction of CD4 T cell responses in nasal associated lymphoid tissue during group A streptococcal infection. Eur. J. Immunol. 34, 2843–2853 (2004).
Hikono, H. et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 204, 1625–1636 (2007).
Snyder, C.M. et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29, 650–659 (2008).
Hendriks, J. et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 1, 433–440 (2000).
Hendriks, J., Xiao, Y. & Borst, J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 198, 1369–1380 (2003).
Dolfi, D.V. et al. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J. Immunol. 180, 2912–2921 (2008).
Rubinstein, M.P. et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc. Natl. Acad. Sci. USA 103, 9166–9171 (2006).
Tokoyoda, K. et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 30, 721–730 (2009).
Weaver, C.T., Harrington, L.E., Mangan, P.R., Gavrieli, M. & Murphy, K.M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 (2006).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Sato, A. et al. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J. Immunol. 171, 3684–3690 (2003).
Kelsall, B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunol. 1, 460–469 (2008).
Reis e Sousa, C. et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186, 1819–1829 (1997).
Hsieh, C.S., Heimberger, A.B., Gold, J.S. & O'Garra, A. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260, 547–549 (1992).
Homann, D., Teyton, L. & Oldstone, M.B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7, 913–919 (2001).
Monack, D.M., Mueller, A. & Falkow, S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2, 747–765 (2004).
Surh, C.D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
Allam, A. et al. The CD8+ memory T-cell state of readiness is actively maintained and reversible. Blood 114, 2121–2130 (2009).
Kraus, Z.J., Haring, J.S. & Bishop, G.A. TNF receptor-associated factor 5 is required for optimal T cell expansion and survival in response to infection. J. Immunol. 181, 7800–7809 (2008).
Intlekofer, A.M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Joshi, N.S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Soares, H. et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 204, 1095–1106 (2007).
Keller, A.M., Schildknecht, A., Xiao, Y., van den Broek, M. & Borst, J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity 29, 934–946 (2008).
van Oosterwijk, M.F. et al. CD27–CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int. Immunol. 19, 713–718 (2007).
Yang, X.O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008).
Ribot, J.C. et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat. Immunol. 10, 427–436 (2009).
Lu, Y.J. et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4, e1000159 (2008).
Yamanaka, N., Hotomi, M. & Billal, D.S. Clinical bacteriology and immunology in acute otitis media in children. J. Infect. Chemother. 14, 180–187 (2008).
Stoklasek, T.A., Schluns, K.S. & Lefrancois, L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177, 6072–6080 (2006).
Boggs, D.R. The total marrow mass of the mouse: a simplified method of measurement. Am. J. Hematol. 16, 277–286 (1984).
Acknowledgements
We thank S. Jameson, H. Chu and J. Taylor for reviewing the manuscript; J. Walter, J. McLachlan and A. Schmidt for technical assistance; and P. Champoux and N. Shah for flow cytometry sorting. Supported by the US National Institutes of Health (AI39614, AI66016 and AI27998 to M.K.J.; T32-AI07313 to A.J.P.; and T32-CA9138 to M.P.).
Author information
Authors and Affiliations
Contributions
M.P. designed the study, did experiments, analyzed data and wrote the manuscript; J.L.L., A.J.P., T.Z. and T.D. did experiments; P.P.C. designed experiments; and M.K.J. designed the study, analyzed data and wrote the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Pepper, M., Linehan, J., Pagán, A. et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol 11, 83–89 (2010). https://doi.org/10.1038/ni.1826
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1826
This article is cited by
-
Establishment of isotype-switched, antigen-specific B cells in multiple mucosal tissues using non-mucosal immunization
npj Vaccines (2023)
-
CD4+ T cell memory
Nature Immunology (2023)
-
Whipworm secretions and their roles in host-parasite interactions
Parasites & Vectors (2022)
-
Regulation of tissue-resident memory T cells by the Microbiota
Mucosal Immunology (2022)
-
Th1 responses in vivo require cell-specific provision of OX40L dictated by environmental cues
Nature Communications (2020)