Abstract
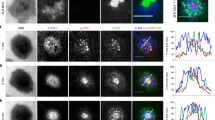
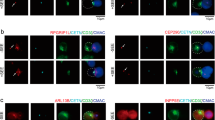
The immunological synapse (IS) is a cell–cell junction formed between CD4+ T cells and dendritic cells (DCs). Here we show in vitro and in vivo that IS formation inhibits apoptosis of DCs. Consistent with these results, IS formation induced antiapoptotic signaling events, including activation of the kinase Akt1 and localization of the prosurvival transcription factor NF-κB and the proapoptotic transcription factor FOXO1 to the nucleus and cytoplasm, respectively. Inhibition of phosphatidylinositol 3-OH kinase and Akt1 partially prevented the antiapoptotic effects of IS formation. Direct stimulation of the IS component CD40 on DCs leads to the activation of Akt1, suggesting the involvement of this receptor in the antiapoptotic effects observed upon IS formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
11 February 2010
In the version of this article initially published, a citation was omitted. It should be cited in the third paragraph of the Discussion section after the second sentence as follows: In this context, it has been suggested that Notch1, another receptor located at the IS(DC), may inhibit the apoptosis of DCs by inducing activation of Akt and STAT3, a transcription factor that promotes cell survival51. The bibliographic information is as follows: 51. Luty, W.H., Rodeberg, D., Parness, J. & Vyas, YM. Antiparallel segregation of notch components in the immunological synapse directs reciprocal signaling in allogeneic Th:DC conjugates. J. Immunol. 179, 819–829 (2007).
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Kupfer, A. & Kupfer, H. Imaging immune cell interactions and functions: SMAC and the immunological synapse. Semin. Immunol. 15, 295–300 (2003).
Rodriguez-Fernandez, J.L. & Corbi, A.L. Adhesion molecules in human dendritic cells. Curr. Opin. Investig. Drugs 6, 1103–1111 (2005).
Stoll, S., Delon, J., Brotz, T.M. & Germain, R.M. Dynamic imaging of T-cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876 (2002).
Delon, J., Stoll, S. & Germain, R.M. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol. Rev. 189, 51–63 (2002).
Huppa, J.B., Gleimer, M., Sumen, C. & Davis, M.M. Continuous T-cell receptor signaling required for synapse maintenance and full effector potential. Nat. Immunol. 4, 749–755 (2003).
Mempel, T.R., Henrickson, S.E. & von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Benvenuti, F. et al. Dendritic cell maturation controls adhesion, synapse formation, and duration of the duration of the interactions with naive T lymphocytes. J. Immunol. 172, 292–301 (2004).
Boisvert, J., Edmondson, S. & Krummel, M.F. Immunological synapse formation licences CD40–CD40L accumulation at T-APC contact sites. J. Immunol. 173, 3647–3652 (2004).
Van der Merwe, P.A., Davis, S.J., Shaw, A.S. & Dustin, M.L. Cytoskeletal polarization and redistribution of cell surface molecules during T cell antigen recognition. Semin. Immunol. 12, 5–21 (2000).
Dustin, M.L., Bromley, S.K., Davis, M.M. & Zhu, C. Identification of self through two-dimensional chemistry and synapses. Annu. Rev. Cell Dev. Biol. 17, 133–157 (2001).
Van der Merwe, P.A. Formation and function of the immunological synapse. Curr. Opin. Immunol. 1, 293–298 (2002).
Davis, D.M. & Dustin, M.L. What is the importance of the immunological synapse? Trends Immunol. 25, 323–327 (2004).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Lee, K.-H. et al. The immunological synapse balances T cell receptor signaling and degradation. Science 302, 1218–1222 (2003).
Davis, S.J. & Van der Merwe, P.A. The immunological synapse: required for T-cell receptor signaling or directing T-cell effector function? Curr. Biol. 11, R289–R291 (2001).
Maldonado, R.A., Irvine, D.J., Schreiber, R. & Glimcher, L.H. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature 431, 527–532 (2004).
Huppa, J.B. & Davis, M.M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 (2003).
Al-Alwan, M.M., Rowden, G., Lee, T.D.G. & West, K.A. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J. Immunol. 166, 1452–1456 (2001).
Strasser, A., O`Connor, L. & Dixit, V.M. Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000).
Hildeman, D., Jorgensen, T., Kappler, J. & Marrack, P. Apoptosis and the homeostatic control of immune responses. Curr. Opin. Immunol. 19, 516–521 (2007).
Downward, J. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 15, 177–182 (2004).
Hou, W.S. & Van Parijs, L.A. Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 5, 583–589 (2004).
Karin, M. & Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 3, 221–227 (2002).
Burgering, B.M.T. & Kops, G.J.P.L. Cell cycle and death control: long lived forkheads. Trends Biochem. Sci. 27, 352–360 (2002).
Birkenkamp, K.U. & Coffer, P.J. FOXO transcription factors as regulators of immune homeostasis: molecules to die for? J. Immunol. 171, 1623–1629 (2003).
Ruedl, C., Koebel, P., Bachmann, M., Hess, M. & Karjalainen, K. Anatomical origin of dendritic cells determine their life span in peripheral lymph nodes. J. Immunol. 165, 4910–4916 (2000).
Garg, S. et al. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin derived dendritic cells in vivo. Nat. Immunol. 4, 907–912 (2003).
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigen stimulation determines the fate of naïve and effector cells. Immunity 8, 89–95 (1998).
Celli, S., Lemaître, F. & Bousso, P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity 27, 625–634 (2007).
Sanchez-Sanchez, N. et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood 104, 619–625 (2004).
Sabatos, C.A. et al. A synapsis basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity 29, 238–248 (2008).
Pozarowski, P. et al. Interactions of fluorochrome-labeled caspases inhibitors with apoptotic cells: a caution in data interpretation. Cytometry A 55, 50–60 (2003).
Lovborg, H., Nygren, P. & Larsson, R. Multiparametric evaluation of apoptosis: effects of standard cytotoxic agents and cyanoguanidine CHS 828. Mol. Cancer Ther. 3, 521–526 (2004).
Várnai, P. & Balla, T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143, 501–510 (1998).
Costello, P.S., Gallagher, P.J. & Cantrell, D.A. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat. Immunol. 3, 1082–1089 (2002).
Vlahakis, S.R. et al. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J. Immunol. 169, 5546–5554 (2002).
Schmid, J.A. et al. Dynamics of NF κB and IκBα studied with green fluorescent protein (GFP) fusion proteins. Investigation of GFP-p65 binding to DNA by fluorescence resonance energy transfer. J. Biol. Chem. 275, 17035–17042 (2000).
Saccani, S., Pantano, S. & Natoli, G. Modulation of NF-κB activity by exchange of dimers. Mol. Cell 11, 1563–1574 (2003).
Chen, G. & Goeddel, D.V. TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635 (2002).
Zhang, X. et al. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J. Biol. Chem. 277, 45276–45284 (2002).
Schreiber, E., Matthias, P., Muller, M.M. & Schaffner, W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 17, 6419 (1989).
Kim, K.D., Choe, Y.K., Choe, I.S. & Lim, J.S. Inhibition of glucocorticoid-mediated, caspase-independent dendritic cell death by CD40 activation. J. Leukoc. Biol. 69, 426–434 (2001).
Miga, A.J. et al. Dendritic cell longevity and T-cell persistence is controlled by CD154–CD40 interactions. Eur. J. Immunol. 31, 959–965 (2001).
Hanks, B.A. et al. Re-engineered CD40 receptor enables potent pharmacological activation of dendritic-cell cancer vaccines in vivo. Nat. Med. 11, 130–137 (2005).
Revy, P., Sospedra, M., Barbour, B. & Trautmann, A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat. Immunol. 2, 925–931 (2001).
Kondo, T. et al. Dendritic cells signal T cells in the absence of exogenous antigen. Nat. Immunol. 2, 932–938 (2001).
Rodriguez-Fernandez, J.L. et al. Rho and ROCK modulate the tyrosine kinase PYK2 in T-cells through regulation of the activity of the integrin LFA-1. J. Biol. Chem. 276, 40518–40527 (2001).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Riol-Blanco, L. et al. The neuronal protein Kidins220 localizes in a raft compartment at the leading edge of motile immature dendritic cells. Eur. J. Immunol. 34, 108–118 (2004).
Luty, W.H., Rodeberg, D., Parness, J. & Vyas, Y.M. Antiparallel segregation of notch components in the immunological synapse directs reciprocal signaling in allogeneic Th:DC conjugates. J. Immunol. 179, 819–829 (2007).
Acknowledgements
We thank A.L. Corbí and V.S. Martínez for their support, G. de la Rosa for help in the initial stages of this project, C. Ardavín for OTII mice, J. Villarejo and I. Treviño for help in obtaining blood samples, R. Borstein for umbilical cords, J.A. Schmid (Medical University of Vienna) for the p65-GFP construct, T. Balla (US National Institutes of Health) for the PH (Akt)-GFP plasmid, T.G. Unterman for the FOXO1-GFP plasmid, N. Hogg (Cancer Research UK) for anti-LFA-1 α-subunit, P. Lastres for help with the cytometer, A. García-Sánz for discussions and C. Escribano-Díaz for critical reading of the manuscript. Supported by (Ministerio de Educación y Ciencia (BFI-2001-0228 and SAF2005-00801), RETICS Program/Instituto de Salud Carlos III (RIER) (RD08/0075 to J.L.R.-F.), Ministerio de Sanidad (scholarship associated with PI021058 to L.R.-B.) and the Ministerio de Educación y Ciencia of Spain (fellowships FPI to C.D.-M. and FPU to N.S.-S.).
Author information
Authors and Affiliations
Contributions
L.R.-B., C.D.-M. and J.L.R.-F. designed research; L.R.-B., C.D.-M., N.S.-S., L.M.A.-C. and G.M.d.H. performed research; L.M.A.-C., M.D.G.-L., G.M.d.H., J.N., F.S.-M., P.S.-M. and C.C., provided analytical tools; L.R.-B., C.D.-M. and J.L.R.-F. analyzed data; J.L.R.-F. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1235 kb)
Rights and permissions
About this article
Cite this article
Riol-Blanco, L., Delgado-Martín, C., Sánchez-Sánchez, N. et al. Immunological synapse formation inhibits, via NF-κB and FOXO1, the apoptosis of dendritic cells. Nat Immunol 10, 753–760 (2009). https://doi.org/10.1038/ni.1750
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1750
This article is cited by
-
Immune synapse formation promotes lipid peroxidation and MHC-I upregulation in licensed dendritic cells for efficient priming of CD8+ T cells
Nature Communications (2023)
-
Mechanisms of CD40-dependent cDC1 licensing beyond costimulation
Nature Immunology (2022)
-
Oral Pathobiont Activates Anti-Apoptotic Pathway, Promoting both Immune Suppression and Oncogenic Cell Proliferation
Scientific Reports (2018)
-
Human IDO-competent, long-lived immunoregulatory dendritic cells induced by intracellular pathogen, and their fate in humanized mice
Scientific Reports (2017)
-
Detecting apoptosis of leukocytes in mouse lymph nodes
Nature Protocols (2014)