Abstract
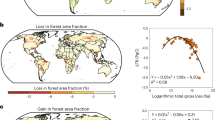
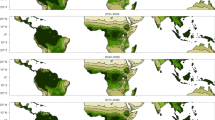
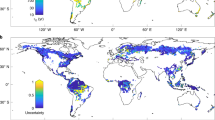
The terrestrial carbon cycle is not well quantified1. Biomass turnover time is a crucial parameter in the global carbon cycle2,3,4, and contributes to the feedback between the terrestrial carbon cycle and climate2,3,4,5,6,7. Biomass turnover time varies substantially in time and space, but its determinants are not well known8,9, making predictions of future global carbon cycle dynamics uncertain5,10,11,12,13. Land use—the sum of activities that aim at enhancing terrestrial ecosystem services14—alters plant growth15 and reduces biomass stocks16, and is hence expected to affect biomass turnover. Here we explore land-use-induced alterations of biomass turnover at the global scale by comparing the biomass turnover of the actual vegetation with that of a hypothetical vegetation state with no land use under current climate conditions. We find that, in the global average, biomass turnover is 1.9 times faster with land use. This acceleration affects all biomes roughly equally, but with large differences between land-use types. Land conversion, for example from forests to agricultural fields, is responsible for 59% of the acceleration; the use of forests and natural grazing land accounts for 26% and 15% respectively. Reductions in biomass stocks are partly compensated by reductions in net primary productivity. We conclude that land use significantly and systematically affects the fundamental trade-off between carbon turnover and carbon stocks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bloom, A. A., Exbrayat, J.-F., Velde, I. R., van der Feng, L. & Williams, M. The decadal state of the terrestrial carbon cycle: global retrievals of terrestrial carbon allocation, pools, and residence times. Proc. Natl Acad. Sci. USA 113, 1285–1290 (2016).
Körner, C. Biosphere responses to CO2 enrichment. Ecol. Appl. 10, 1590–1619 (2000).
Odum, E. P. Fundamentals of Ecology Vol. 3 (Saunders, 1971).
Saugier, B., Roy, J. & Mooney, H. A. in Terrestrial Global Productivity (eds Roy, J., Saugier, B. & Mooney, H. A.) 543–557 (Academic, 2001).
Malhi, Y. The productivity, metabolism and carbon cycle of tropical forest vegetation. J. Ecol. 100, 65–75 (2012).
Körner, C. et al. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2 . Science 309, 1360–1362 (2005).
Barrett, D. J. Steady state turnover time of carbon in the Australian terrestrial biosphere. Glob. Biogeochem. Cycles 16, 55 (2002).
Carvalhais, N. et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 514, 213–217 (2014).
Keeling, H. C. & Phillips, O. L. The global relationship between forest productivity and biomass. Glob. Ecol. Biogeogr. 16, 618–631 (2007).
Friend, A. D. et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2 . Proc. Natl Acad. Sci. USA 111, 3280–3285 (2014).
Negrón-Juárez, R. I., Koven, C. D., Riley, W. J., Knox, R. G. & Chambers, J. Q. Observed allocations of productivity and biomass, and turnover times in tropical forests are not accurately represented in CMIP5 Earth system models. Environ. Res. Lett. 10, 64017 (2015).
Delbart, N. et al. Mortality as a key driver of the spatial distribution of aboveground biomass in Amazonian forest: results from a dynamic vegetation model. Biogeosciences 7, 3027–3039 (2010).
Wang, W. et al. Diagnosing and assessing uncertainties of terrestrial ecosystem models in a multimodel ensemble experiment: 2. Carbon balance. Glob. Change Biol. 17, 1367–1378 (2011).
Erb, K.-H. et al. A comprehensive global 5 min resolution land-use data set for the year 2000 consistent with national census data. J. Land Use Sci. 2, 191–224 (2007).
Haberl, H., Erb, K.-H. & Krausmann, F. Human appropriation of net primary production: patterns, trends, and planetary boundaries. Annu. Rev. Environ. Res. 39, 363–391 (2014).
Egglestone, H. S., Buendia, L., Miwa, K. & Ngara, T. IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme (IGES, 2006).
Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 25, 107–114 (2015).
Galbraith, D. et al. Residence times of woody biomass in tropical forests. Plant Ecol. Diver. 6, 139–157 (2013).
Ahlström, A., Xia, J., Arneth, A., Luo, Y. & Smith, B. Importance of vegetation dynamics for future terrestrial carbon cycling. Environ. Res. Lett. 10, 54019 (2015).
Haberl, H. et al. Quantifying and mapping the human appropriation of net primary production in Earth’s terrestrial ecosystems. Proc. Natl Acad. Sci. USA 104, 12942–12947 (2007).
Krausmann, F. et al. Global human appropriation of net primary production doubled in the 20th century. Proc. Natl Acad. Sci. USA 110, 10324–10329 (2013).
Luyssaert, S. et al. Land management and land-cover change have impacts of similar magnitude on surface temperature. Nature Clim. Change 4, 389–393 (2014).
DeFries, R. Past and future sensitivity of primary production to human modification of the landscape. Geophys. Res. Lett. 29, 1132 (2002).
Pongratz, J., Reick, C., Raddatz, T. & Claussen, M. Effects of anthropogenic land cover change on the carbon cycle of the last millennium. Glob. Biogeochem. Cycles 23, GB4001 (2009).
Don, A., Schumacher, J. & Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—a meta-analysis. Glob. Change Biol. 17, 1658–1670 (2011).
Global Forest Resources Assessment 2010 Main Report (FAO, 2010).
Steffen, W., Broadgate, W., Deutsch, L., Gaffney, O. & Ludwig, C. The trajectory of the Anthropocene: the great acceleration. Anthrop. Rev. 2, 81–98 (2015).
Tilman, D., Balzer, C., Hill, J. & Befort, B. L. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA 108, 20260–20264 (2011).
Campioli, M. et al. Biomass production efficiency controlled by management in temperate and boreal ecosystems. Nature Geosci. 8, 843–846 (2015).
Thurner, M. et al. Carbon stock and density of northern boreal and temperate forests. Glob. Ecol. Biogeogr. 23, 297–310 (2014).
Olson, D. M. et al. Terrestrial ecoregions of the world: a new map of life on Earth a new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51, 933–938 (2001).
Luyssaert, S. et al. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob. Change Biol. 13, 2509–2537 (2007).
Bond-Lamberty, B. & Thomson, A. A global database of soil respiration data. Biogeosciences 7, 1915–1926 (2010).
Noormets, A. et al. Effects of forest management on productivity and carbon sequestration: a review and hypothesis. Forest Ecol. Manag. 355, 124–140 (2015).
Michaletz, S. T., Cheng, D., Kerkhoff, A. J. & Enquist, B. J. Convergence of terrestrial plant production across global climate gradients. Nature 512, 39–43 (2014).
Fang, J. et al. Overestimated biomass carbon pools of the northern mid- and high latitude forests. Clim. Change 74, 355–368 (2006).
Brown, S. & Lugo, A. E. Biomass of tropical forests: a new estimate based on forest volumes. Science 223, 1290–1293 (1984).
Brown, S. Estimating Biomass and Biomass Change of Tropical Forests: A Primer (Food & Agriculture Org., 1997).
Bartholomé, E. & Belward, A. S. GLC2000: a new approach to global land cover mapping from Earth observation data. Int. J. Remote Sens. 26, 1959–1977 (2005).
Sanderson, E. W. et al. The human footprint and the last of the wild. BioScience 52, 891–904 (2002).
Statistical Databases (FAOSTAT, accessed 13 October 2014); http://faostat.fao.org
FAO Global Ecological Zoning for the Global Forest Resources Assessment, 2000 (Food and Agriculture Organization of the United Nations, 2001).
Ramankutty, N. & Foley, J. A. Estimating historical changes in global land cover: croplands from 1700 to 1992. Glob. Biogeochem. Cycles 13, 997–1027 (1999).
DiMiceli, C. M. et al. Vegetation Continuous Fields MOD44B 20011 Percent Tree Cover, Collection 5. (University of Maryland, accessed 10 October 2014); http://glcf.umd.edu/data/vcf
Lieth, H. Primary Productivity of the Biosphere 237–263 (Springer, 1975).
Bondeau, A. et al. Modelling the role of agriculture for the 20th century global terrestrial carbon balance. Glob. Change Biol. 13, 679–706 (2007).
Gerten, D., Schaphoff, S., Haberlandt, U., Lucht, W. & Sitch, S. Terrestrial vegetation and water balance—hydrological evaluation of a dynamic global vegetation model. J. Hydrol. 286, 249–270 (2004).
Sitch, S. et al. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs). Glob. Change Biol. 14, 2015–2039 (2008).
Ciais, P. et al. The European carbon balance. Part 2: croplands. Glob. Change Biol. 16, 1409–1428 (2010).
Krausmann, F., Erb, K.-H., Gingrich, S., Lauk, C. & Haberl, H. Global patterns of socioeconomic biomass flows in the year 2000: a comprehensive assessment of supply, consumption and constraints. Ecol. Econ. 65, 471–487 (2008).
Oldeman, L. R., Hakkeling, R. T. A. & Sombrock, W. G. World Map of the Status of Human—Induced Soil Degradation (ISRIC Wageningen, 1991).
Zika, M. & Erb, K. H. The global loss of net primary production resulting from human-induced soil degradation in drylands. Ecol. Econ. 69, 310–318 (2009).
Haberl, H. et al. Changes in ecosystem processes induced by land use: human appropriation of aboveground NPP and its influence on standing crop in Austria. Glob. Biogeochem. Cycles 15, 929–942 (2001).
O’Neill, D. W., Tyedmers, P. H. & Beazley, K. F. Human appropriation of net primary production (HANPP) in Nova Scotia, Canada. Reg. Environ. Change 7, 1–14 (2006).
Harmon, M. E., Ferrell, W. K. & Franklin, J. F. Effects on carbon storage of conversion of old-growth forests to young forests. Science 247, 699–702 (1990).
Ryan, M. G., Binkley, D. & Fownes, J. H. Age-related decline in forest productivity. Adv. Ecol. Res. 27, 213–262 (1997).
Zaehle, S. et al. The importance of age-related decline in forest NPP for modeling regional carbon balances. Ecol. Appl. 16, 1555–1574 (2006).
Saikku, L., Mattila, T., Akujärvi, A. & Liski, J. Human appropriation of net primary production in Finland during 1990–2010. Biomass Bioenergy 83, 559–567 (2015).
Larocque, G. R. Ecological Forest Management Handbook (CRC, 2016).
Olson, J. S., Watts, J. A. & Allison, L. J. Carbon in Live Vegetation of Major World Ecosystems (Oak Ridge National Laboratory, 1983).
Cannell, M. G. R. World Forest Biomass and Primary Production Data 67 (Academic, 1982).
Ajtay, G. L., Ketner, P. & Duvigneaud, P. The Global Carbon Cycle. SCOPE 13 129–182 (Wiley, 1979).
Ruesch, A. & Gibbs, H. K. New IPCC Tier-1 Global Biomass Carbon Map for the Year 2000 (Oak Ridge National Laboratory, accessed 15 January 2015); http://cdiac.ornl.gov/epubs/ndp/global_carbon/carbon_documentation.html
Amthor, J. S. et al. Boreal forest CO2 exchange and evapotranspiration predicted by nine ecosystem process models: intermodel comparisons and relationships to field measurements. J. Geophys. Res. 106, 33623–33648 (2001).
Gower, S. T. et al. Carbon distribution and aboveground net primary production in aspen, jack pine, and black spruce stands in Saskatchewan and Manitoba, Canada. J. Geophys. Res. 102, 29029–29041 (1997).
Jarvis, P. G., Saugier, B. & Schulze, E.-D. in Terrestrial Global Productivity (eds Roy, J., Saugier, B. & Mooney, H. A.) 211–244 (Academic, 2001).
Pan, Y. et al. A large and persistent carbon sink in the World’s forests. Science 333, 988–993 (2011).
Kauppi, P. E. New, low estimate for carbon stock in global forest vegetation based on inventory data. Silva Fenn. 37, 451–457 (2003).
MacDicken, K. G. Global Forest Resources Assessment 2015: What, why and how? Forest Ecol. Manag. 352, 3–8 (2015).
Clark, D. B. & Kellner, J. R. Tropical forest biomass estimation and the fallacy of misplaced concreteness. J. Veg. Sci. 23, 1191–1196 (2012).
Houghton, R. A., Lawrence, K. T., Hackler, J. L. & Brown, S. The spatial distribution of forest biomass in the Brazilian Amazon: a comparison of estimates. Glob. Change Biol. 7, 731–746 (2001).
Simard, M., Pinto, N., Fisher, J. B. & Baccini, A. Mapping forest canopy height globally with spaceborne lidar. J. Geophys. Res. 116, G04021 (2011).
Nogueira, E. M., Fearnside, P. M., Nelson, B. W., Barbosa, R. I. & Keizer, E. W. H. Estimates of forest biomass in the Brazilian Amazon: new allometric equations and adjustments to biomass from wood-volume inventories. Forest Ecol. Manag. 256, 1853–1867 (2008).
Feldpausch, T. R. et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences 9, 3381–3403 (2012).
Chave, J. et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 20, 3177–3190 (2014).
Lefsky, M. A. et al. Lidar remote sensing of above-ground biomass in three biomes. Global Ecol. Biogeogr. 11, 393–399 (2002).
Drake, J. B. et al. Estimation of tropical forest structural characteristics using large-footprint lidar. Remote Sens. Environ. 79, 305–319 (2002).
Asner, G. P. High-resolution forest carbon stocks and emissions in the Amazon. Proc. Natl Acad. Sci. USA 107, 16738–16742 (2010).
Mitchard, E. T. et al. Uncertainty in the spatial distribution of tropical forest biomass: a comparison of pan-tropical maps. Carbon Balance Manag. 8, 10 (2013).
Mitchard, E. T. A. et al. Markedly divergent estimates of Amazon forest carbon density from ground plots and satellites. Glob. Ecol. Biogeogr. 23, 935–946 (2014).
Saatchi, S. et al. Seeing the forest beyond the trees. Glob. Ecol. Biogeogr. 24, 606–610 (2015).
Kindermann, G. E., McCallum, I., Fritz, S. & Obersteiner, M. A global forest growing stock, biomass and carbon map based on FAO statistics. Silva Fenn. 42, 387–396 (2008).
Bolin, B. in The Greenhouse Effect, Climatic Change, and Ecosystems. SCOPE 29 (eds Bolin, B., Döös, B. R., Jäger, J. & Warrick, R. A.) 93–155 (Wiley, 1986).
Pan, Y., Birdsey, R. A., Phillips, O. L. & Jackson, R. B. The structure, distribution, and biomass of the World’s forests. Annu. Rev. Ecol. Evol. Syst. 44, 593–622 (2013).
Baccini, A. et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nature Clim. Change 2, 182–185 (2012).
Saatchi, S. S. et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl Acad. Sci. USA 108, 9899–9904 (2011).
Zhao, M., Heinsch, F. A., Nemani, R. R. & Running, S. W. Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens. Environ. 95, 164–176 (2005).
West, P. C. et al. Trading carbon for food: global comparison of carbon stocks versus crop yields on agricultural land. Proc. Natl Acad. Sci. USA 107, 19645–19648 (2010).
Ang, B. W. The LMDI approach to decomposition analysis: a practical guide. Energy Policy 33, 867–871 (2005).
Acknowledgements
The authors gratefully acknowledge funding from the European Research Council (ERC-2010-stg-263522 ‘LUISE’), the European Commission (H2020-EO-2014-640176 ‘BACI’), and the ProVision Programme of the Austrian Ministry of Science. We thank M. Thurner for providing the temperate–boreal woody carbon stock data set (ref. 30). This research contributes to the Global Land Project (www.globallandproject.org).
Author information
Authors and Affiliations
Contributions
K.-H.E., T.F., C.P. and H.H. designed the study. K.-H.E., T.F., T.K. and C.P. performed the empirical research. All authors contributed significantly to the final analysis, interpretation or results and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1178 kb)
Rights and permissions
About this article
Cite this article
Erb, KH., Fetzel, T., Plutzar, C. et al. Biomass turnover time in terrestrial ecosystems halved by land use. Nature Geosci 9, 674–678 (2016). https://doi.org/10.1038/ngeo2782
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2782
This article is cited by
-
A biomass map of the Brazilian Amazon from multisource remote sensing
Scientific Data (2023)
-
The impact of climate change and human activities on the change in the net primary productivity of vegetation—taking Sichuan Province as an example
Environmental Science and Pollution Research (2023)
-
Assessing carbon cycle projections from complex and simple models under SSP scenarios
Climatic Change (2023)
-
The global carbon sink potential of terrestrial vegetation can be increased substantially by optimal land management
Communications Earth & Environment (2022)
-
Quantifying the impacts of land cover change on gross primary productivity globally
Scientific Reports (2022)