Abstract
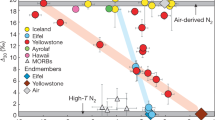
Volatile elements stored in the mantles of terrestrial planets escape through volcanic degassing, and thereby influence planetary atmospheric evolution and habitability. Compared with the atmospheres of Venus and Mars, Earth's atmosphere is nitrogen-rich relative to primordial noble gas concentrations1,2,3. The compatibility of volatile elements in mantle minerals versus melts and fluids controls how readily these elements are degassed. However, the speciation of nitrogen in mantle fluids is not well constrained4,5,6. Here we present thermodynamic calculations that establish the speciation of nitrogen in aqueous fluids under upper mantle conditions. We find that, under the relatively oxidized conditions of Earth's mantle wedges at convergent plate margins7,8,9, nitrogen is expected to exist predominantly as N2 in fluids and, therefore, be degassed easily. In contrast, under more reducing conditions elsewhere in the Earth's upper mantle and in the mantles of Venus and Mars, nitrogen is expected predominantly in the form of ammonium (NH4+) in aqueous fluids. Ammonium is moderately compatible in upper mantle minerals10,11 and unconducive to nitrogen degassing. We conclude that Earth's oxidized mantle wedge conditions—a result of subduction and hence plate tectonics—favour the development of a nitrogen-enriched atmosphere, relative to the primordial noble gases, whereas the atmospheres of Venus and Mars have less nitrogen because they lack plate tectonics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Porcelli, D. & Pepin, R. O. in Treatise on Geochemistry. Volume 4: The Atmosphere (eds Holland, H. D. & Turekian, K. K.) 319–347 (2003).
Mahaffy, P. R. et al. Abundance and isotopic composition of gases in the Martian atmosphere from the Curiosity Rover. Science 341, 263–266 (2013).
Hoffmann, J. H., Oyama, V. I. & Zahn, U. V. Measurements of the lower atmospheric composition: A comparison of results. J. Geophys. Res. 85, 7871–7881 (1980).
Busigny, V. & Bebout, G. E. Nitrogen in the silicate Earth: Speciation and isotopic behaviour during mineral–fluid interactions. Elements 9, 353–358 (2013).
Li, Y. & Keppler, H. Nitrogen speciation in mantle and crustal fluids. Geochim. Cosmochim. Acta 129, 13–32 (2014).
Mysen, B. O., Tomita, T., Ohtani, E. & Suzuki, A. Speciation of and D/H partitioning between fluids and melts in silicate–D–O–H–C–N systems determined in situ at upper mantle temperatures, pressures, and redox conditions. Am. Mineral. 99, 578–588 (2014).
Frost, D. J. & McCammon, C. M. The redox state of Earth's mantle. Annu. Rev. Earth Planet. Sci. 36, 389–420 (2008).
Wood, B. J., Bryndzia, L. T. & Johnson, K. E. Mantle oxidation state and its relationship to tectonic environment and fluid speciation. Science 248, 337–345 (1990).
Parkinson, I. J. & Arculus, R. J. The redox state of subduction zones: Insights from arc-peridotites. Chem. Geol. 160, 409–423 (1999).
Watenphul, A., Wunder, B., Wirth, R. & Heinrich, W. Ammonium-bearing clinopyroxene: A potential nitrogen reservoir in the Earth's mantle. Chem. Geol. 270, 240–248 (2010).
Li, Y., Wiedenbeck, M., Shcheka, S. & Keppler, H. Nitrogen solubility in upper mantle minerals. Earth Planet. Sci. Lett. 377–378, 311–323 (2013).
McCubbin, F. M. et al. Hydrous melting of the martian mantle produced both depleted and enriched shergottites. Geology 40, 683–686 (2012).
Busigny, V., Cartigny, P. & Philippot, P. Nitrogen isotopes in ophiolitic metagabbros: A re-evaluation of modern nitrogen fluxes in subduction zones and implication for the early Earth atmosphere. Geochim. Cosmochim. Acta 75, 7502–7521 (2011).
Roskosz, M., Bouhifd, M. A., Jephcoat, A. P., Marty, B. & Mysen, B. O. Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochim. Cosmochim. Acta 121, 15–28 (2013).
Marty, B. The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313–314, 56–66 (2012).
Bebout, G. E., Fogel, M. L. & Cartigny, P. Nitrogen: Highly volatile yet surprisingly compatible. Elements 9, 333–338 (2013).
Shcheka, S. S. & Keppler, H. The origin of the terrestrial noble-gas signature. Nature 490, 531–536 (2012).
Pan, D., Spanu, L., Harrison, B., Sverjensky, D. A. & Galli, G. Dielectric properties of water under extreme conditions and transport of carbonates in the deep Earth. Proc. Natl Acad. Sci. USA 110, 6646–6650 (2013).
Facq, S., Daniel, I., Montagnac, G., Cardon, H. & Sverjensky, D. A. In situ Raman study and thermodynamic model of aqueous carbonate speciation in equilibrium with aragonite under subduction zone conditions. Geochim. Cosmochim. Acta 132, 375–390 (2014).
Sverjensky, D. A., Harrison, B. & Azzolini, D. Water in the deep Earth: the dielectric constant and the solubilities of quartz and corundum to 60 kb and 1,200 °C. Geochim. Cosmochim. Acta 129, 125–145 (2014).
Herd, C. D. K. Basalts as probes of planetary interior redox state. Rev. Min. Geochem. 68, 527–553 (2008).
Lecuyer, C. & Ricard, Y. Long-term fluxes and budget of ferric iron: Implication for the redox states of the Earth's mantle and atmosphere. Earth Planet. Sci. Lett. 165, 197–211 (1999).
Kelley, K. A. & Cottrell, E. Water and the oxidation state of subduction zone magmas. Science 325, 605–609 (2009).
Marty, B., Zimmermann, L., Pujol, M., Burgess, R. & Philippot, P. Nitrogen isotopic composition and density of the Archean atmosphere. Science 342, 101–104 (2013).
Turner, S., Rushmer, T., Reagan, M. & Moyen, J-F. Heading down early on? Start of subduction on Earth. Geology 42, 139–142 (2014).
Holland, H. D. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. Lond. B 361, 903–915 (2006).
Hirschmann, M. Ironing out the oxidation of Earth's mantle. Science 325, 545–546 (2009).
Fischer, T. P. et al. Subduction and recycling of nitrogen along the Central American margin. Science 297, 1154–1157 (2002).
Yokochi, R., Marty, B., Chazot, G. & Burnard, P. Nitrogen in peridotite xenoliths: Lithophile behavior and magmatic isotope fractionation. Geochim. Cosmochim. Acta 73, 4843–4861 (2009).
Quintana, E. V. et al. An Earth-sized planet in the habitable zone of a cool star. Science 344, 277–280 (2014).
Acknowledgements
This research was supported by a Carnegie Postdoctoral Fellowship, grants from the Sloan Foundation through the Deep Carbon Observatory (Reservoirs and Fluxes, and Extreme Physics and Chemistry programs) and grants DOE DE-FG-02-96ER-14616 and NSF EAR 1023865. We are also grateful for the help and support of the Johns Hopkins University and the Geophysical Laboratory of the Carnegie Institution of Washington. We wish to acknowledge reviews of the manuscript by R. W. Carlson, R. M. Hazen, J. Hopp and B. Marty, as well as helpful discussions with B. Mysen and C. Schiffries (CIW).
Author information
Authors and Affiliations
Contributions
S.M. conceived the idea and collated the planetary datasets, D.A.S. performed the thermodynamic calculations, and both authors contributed equally to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1186 kb)
Rights and permissions
About this article
Cite this article
Mikhail, S., Sverjensky, D. Nitrogen speciation in upper mantle fluids and the origin of Earth's nitrogen-rich atmosphere. Nature Geosci 7, 816–819 (2014). https://doi.org/10.1038/ngeo2271
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2271
This article is cited by
-
Temperature dependence of nitrogen solubility in bridgmanite and evolution of nitrogen storage capacity in the lower mantle
Scientific Reports (2023)
-
A primary magmatic source of nitrogen to Earth’s crust
Nature Geoscience (2023)
-
Plate Tectonics: The Stabilizer of Earth’s Habitability
Journal of Earth Science (2023)
-
Earth’s Nitrogen and Carbon Cycles
Space Science Reviews (2021)
-
Dehydration at subduction zones and the geochemistry of slab fluids
Science China Earth Sciences (2020)