Abstract
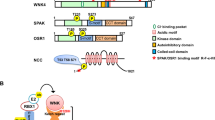
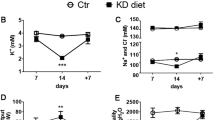
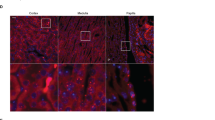
The mechanisms that govern homeostasis of complex systems have been elusive but can be illuminated by mutations that disrupt system behavior. Mutations in the gene encoding the kinase WNK4 cause pseudohypoaldosteronism type II (PHAII), a syndrome featuring hypertension and hyperkalemia. We show that physiology in mice transgenic for genomic segments harboring wild-type (TgWnk4WT) or PHAII mutant (TgWnk4PHAII) Wnk4 is changed in opposite directions: TgWnk4PHAII mice have higher blood pressure, hyperkalemia, hypercalciuria and marked hyperplasia of the distal convoluted tubule (DCT), whereas the opposite is true in TgWnk4WT mice. Genetic deficiency for the Na-Cl cotransporter of the DCT (NCC) reverses phenotypes seen in TgWnk4PHAII mice, demonstrating that the effects of the PHAII mutation are due to altered NCC activity. These findings establish that Wnk4 is a molecular switch that regulates the balance between NaCl reabsorption and K+ secretion by altering the mass and function of the DCT through its effect on NCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lifton, R.P., Gharavi, A.G. & Geller, D.S. Molecular mechanisms of human hypertension. Cell 104, 545–556 (2001).
Paver, W.K. & Pauline, G.J. Hypertension and hyperpotassaemia without renal disease in a young male. Med. J. Aust. 35, 305–306 (1964).
Gordon, R.D. The syndrome of hypertension and hyperkalemia with normal glomerular filtration rate: Gordon's syndrome. Aust. N. Z. J. Med. 16, 183–184 (1986).
Wilson, F.H. et al. Human hypertension caused by mutations in WNK kinases. Science 293, 1107–1112 (2001).
Wilson, F.H. et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc. Natl. Acad. Sci. USA 100, 680–684 (2003).
Yang, C.L., Angell, J., Mitchell, R. & Ellison, D.H. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J. Clin. Invest. 111, 1039–1045 (2003).
Kahle, K.T. et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat. Genet. 35, 372–376 (2003).
Kahle, K.T. et al. Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc. Natl. Acad. Sci. USA 101, 14877–14882 (2004).
Yamauchi, K. et al. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc. Natl. Acad. Sci. USA 101, 4690–4694 (2004).
Kahle, K.T., Wilson, F.H. & Lifton, R.P. Regulation of diverse ion transport pathways by WNK4 kinase: a novel molecular switch. Trends Endocrinol. Metab. 16, 98–103 (2005).
Lalioti, M. & Heath, J. A new method for generating point mutations in bacterial artificial chromosomes by homologous recombination in Escherichia coli. Nucleic Acids Res. 29, E14 (2001).
Kahle, K.T. et al. WNK4 regulates apical and basolateral Cl- flux in extrarenal epithelia. Proc. Natl. Acad. Sci. USA 101, 2064–2069 (2004).
Loffing, J. et al. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am. J. Physiol. Renal Physiol. 281, F1021–F1027 (2001).
Schultheis, P.J. et al. Phenotype resembling Gitelman's syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J. Biol. Chem. 273, 29150–29155 (1998).
Mayan, H. et al. Hypercalciuria in familial hyperkalemia and hypertension accompanies hyperkalemia and precedes hypertension: description of a large family with the Q565E WNK4 mutation. J. Clin. Endocrinol. Metab. 89, 4025–4030 (2004).
Levy, D. et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension 36, 477–483 (2000).
Simon, D.B. et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat. Genet. 12, 24–30 (1996).
Loffing, J. et al. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman's syndrome. J. Am. Soc. Nephrol. 15, 2276–2288 (2004).
Kaissling, B., Bachmann, S. & Kriz, W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am. J. Physiol. 248, F374–F381 (1985).
Nijenhuis, T. et al. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J. Clin. Invest. 115, 1651–1658 (2005).
Yamakawa, H., Suzuki, H., Nakamura, M., Ohno, Y. & Saruta, T. Disturbed calcium metabolism in offspring of hypertensive parents. Hypertension 19, 528–534 (1992).
Lind, L., Lithell, H., Gustafsson, I.B., Pollare, T. & Ljunghall, S. Calcium metabolism and sodium sensitivity in hypertensive subjects. J. Hum. Hypertens. 7, 53–57 (1993).
Chang, S.S. et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat. Genet. 12, 248–253 (1996).
Reilly, R.F. & Ellison, D.H. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol. Rev. 80, 277–313 (2000).
Weinstein, A.M. A mathematical model of rat distal convoluted tubule. I. Cotransporter function in early DCT. Am. J. Physiol. Renal Physiol. 289, F699–F720 (2005).
Rubera, I. et al. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J. Clin. Invest. 112, 554–565 (2003).
Schambelan, M., Sebastian, A. & Rector, F.C., Jr. Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int. 19, 716–727 (1981).
Mujais, S.K., Kauffman, S. & Katz, A.I. Angiotensin II binding sites in individual segments of the rat nephron. J. Clin. Invest. 77, 315–318 (1986).
Wang, T. & Giebisch, G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am. J. Physiol. 271, F143–F149 (1996).
Chrast, R., Scott, H.S. & Antonarakis, S.E. Linearization and purification of BAC DNA for the development of transgenic mice. Transgenic Res. 8, 147–150 (1999).
Brooks, H.L., Allred, A.J., Beutler, K.T., Coffman, T.M. & Knepper, M.A. Targeted proteomic profiling of renal Na(+) transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension 39, 470–473 (2002).
Hansen, P.B. et al. Plasma renin in mice with one or two renin genes. Acta Physiol. Scand. 181, 431–437 (2004).
Kim, G.H. et al. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc. Natl. Acad. Sci. USA 95, 14552–14557 (1998).
Acknowledgements
This work was supported in part by a Human Frontiers Science Program (HFSP) long-term fellowship (M.D.L.; LT00606/2002) and a US National Institutes of Health Specialized Center of Research in Hypertension (R.P.L., D.S.G.). We thank C. Canessa for the anti-β-ENaC and J. Wade and M. Knepper for anti-NCC. We thank A. Louvi and X. Tian for help with cardiac perfusion, C. Spilianakis for the GAPDH BAC, D. Sakkas for help with statistical analysis and H. Qin, K. Choate, K. Finberg and F. Wilson for discussion. We also thank R. Pongratz and L. Rosenthal for electrolyte measurements and S. Mentone for tissue sections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Immunostaining for Wnk4, THP and Ncc in kidney sections of a Tg(Wnk4PHAII) mouse. (PDF 700 kb)
Supplementary Fig. 2
Immunostaining for Ncc. (PDF 620 kb)
Supplementary Fig. 3
Immunostaining for Romk and β-ENaC. (PDF 1162 kb)
Supplementary Fig. 4
Quantitative RT-PCR. (PDF 142 kb)
Supplementary Table 1
Blood pressure. (PDF 57 kb)
Supplementary Table 2
Serum electrolytes. (PDF 63 kb)
Supplementary Table 3
Urine electrolytes. (PDF 36 kb)
Supplementary Table 4
PCR primers. (PDF 27 kb)
Rights and permissions
About this article
Cite this article
Lalioti, M., Zhang, J., Volkman, H. et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38, 1124–1132 (2006). https://doi.org/10.1038/ng1877
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1877
This article is cited by
-
Epigenetic modification: a regulatory mechanism in essential hypertension
Hypertension Research (2019)
-
Overexpression of WNK1 in POMC-expressing neurons reduces weigh gain via WNK4-mediated degradation of Kir6.2
Molecular and Cellular Biochemistry (2018)
-
WNK signalling pathways in blood pressure regulation
Cellular and Molecular Life Sciences (2017)
-
Molecular Mechanisms of Sodium-Sensitive Hypertension in the Metabolic Syndrome
Current Hypertension Reports (2017)
-
Common variants in CLDN14 are associated with differential excretion of magnesium over calcium in urine
Pflügers Archiv - European Journal of Physiology (2017)