Abstract
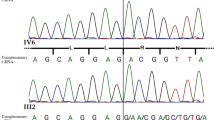
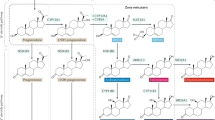
Human male sexual differentiation requires production of fetal testicular testosterone, whose biosynthesis requires steroid 17,20-lyase activity1,2. Patients with putative isolated 17,20-lyase deficiency have been reported3,4. The existence of true isolated 17,20-lyase deficiency, however, has been questioned because 17α-hydroxylase and 17,20-lyase activities are catalyzed by a single enzyme5–8, microsomal cytochrome P450c17, and because the index case of apparent isolated 17,20-lyase deficiency had combined deficiencies of both activities9,10. We studied two patients with clinical and hormonal findings suggestive of isolated 17,20-lyase deficiency. We found two patients homozygous for substitution mutations in CYP17, the gene encoding P450c17. When expressed in COS-1 cells, the mutants retained 17α-hydroxylase activity but had minimal 17,20-lyase activity. Substrate competition experiments suggested that the mutations did not alter the enzyme's substrate-binding capacity, but co-transfection of cells with P450 oxidoreductase, the electron donor used by P450c17, indicated that the mutants had a diminished ability to interact with redox partners. Computer-graphic modelling of P450c17 suggests that both mutations lie in or near the redox-partner binding site, on the opposite side of the haem from the substrate-binding pocket. These mutations alter electrostatic charge distribution in the redox-partner binding site, so that electron transfer for the 17,20-lyase reaction is selectively lost or diverted to uncoupling reactions. These are the first proven cases of isolated 17,20-lyase deficiency, and they demonstrate a novel mechanism for loss of enzymatic activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Grumbach, M.M. & Conte, F.A. Disorders of sexual differentiation. In Williams' Textbook of Endocrinology, 8th ed. (eds Wilson, J.D. & Foster, D.W.) 853–952 (Harcourt Brace, San Diego, California, (1992).
Miller, W.L. & Tyrrell, J.B. The adrenal cortex. In Endocrinology and Metabolism, 3rd ed. (eds Felig, P., Baxter, J. & Frohman, L.) 555–717 (McGraw-Hill, New York, (1995).
Zachman, M., Vollmin, J.A., Hamilton, W. & Prader, A. Steroid 17,20-desmolase deficiency: a new cause of male pseudohermaphroditism. Clin. Endocrinol. 1, 369–385 (1972).
Yanase, T., Simpson, E.R. & Waterman, M.R. 17α-hydroxylase/17,20-lyase deficiency: from clinical investigation to molecular definition. Endocr. Rev. 12, 91–108 (1991).
Nakajin, S., Shively, J.E., Yuan, P. & Hall, P.F. Microsomal cytochrome P450 from neonatal pig testis: two enzymatic activities (17α-hydroxylase and C17,20-lyase) associated with one protein. Biochemistry 20, 4037–4042 (1981).
Nakajin, S., Shinoda, M., Haniu, M., Shively, J.E. & Hall, P.F. C21 steroid side-chain cleavage enzyme from porcine adrenal microsomes: purification and characterization of the 17α-hydroxylase/C17,20 lyase cytochrome P450. J Biol. Chem. 259, 3971–3976 (1984).
Zuber, M.X., Simpson, E.R. & Waterman, M.R. Expression of bovine 17α-hydroxylase cytochrome P450 cDNA in non-steroidogenic (COS-1) cells. Science 234, 1258–1261 (1986).
Lin, D., Harikrishna, J.A., Moore, C.C.D., Jones, K.L. & Miller, W.L. Missense mutation Ser106→Pro causes 17α-hydroxylase deficiency. J. Biol. Chem. 266, 15992–15998 (1991).
Yanase, T. et al. Molecular basis of apparent isolated 17,20-lyase deficiency: compound heterozygous mutations in the C-terminal region (Arg(496)→Cys, Gln(461)→Stop) actually cause combined 17α-hydroxylase/17,20-lyase deficiency. Biochem. Biophys. Acta 1139, 275–279 (1992).
Zachman, M., Kenpken, B., Manella, B. & Navarro, E. Conversion from pure 17,20-desmolase to combined 17,20-desmolase/17α-hydroxylase deficiency with age. Acta Endocrinol. 127, 97–99 (1992).
Picado-Leonard, J. & Miller, W.L. Cloning and sequence of the human gene encoding P450c17 (steroid 17α-hydroxylase/17,20-lyase): similarity to the gene for P450c21. DNA 6, 439–448 (1987).
Sparkes, R.S., Klisak, I. & Miller, W.L. Regional mapping of genes encoding human steroidogenic enzymes: P450scc to 15q23–q24, adrenodoxin to 11q22; adrenodoxin reductase to 17q24–q25; and P450c17 to 10q24–q25. DNA Cell. Biol. 10, 359–365 (1991).
Fan, Y.S. et al. Localization of the human CYP17 gene (cytochrome P450 17α) to 10q24.3 by fluorescence in situ hybridization and simultaneous chromosome banding. Genomics 14, 1110–1111 (1992).
Chung, B. et al. Cytochrome P450c17 (steroid 17α-hydroxylase/17,20-lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl. Acad. Sci. USA 84, 407–411 (1987).
Fardella, C.E., Hum, D.W., Homoki, J. & Miller, W.L. Point mutation Arg440 to His in cytochrome P450c17 causes severe 17α-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 79, 160–164 (1994).
Yanase, T. 17α-hydroxylase/17, 20-lyase defects. J. Steroid Biochem. Mol. Biol. 53, 153–157 (1995).
Bach, G., Moskowitz, S.M., Tieu, P.T., Matynia, A. & Neufeld, E.F. Molecular analysis of Hurler syndrome in Druze and Muslim Arab patients in Israel: multiple allelic mutations of the IDUA gene in a small geographic area. Am. J. Hum. Genet. 53, 330–338 (1993).
Heinisch, U., Zlotogora, J., Kafert, S. & Gieselmann, V. Multiple mutations are responsible for the high frequency of metachromatic leukodystrophy in a small geographic area. Am. J. Hum. Genet. 56, 51–57 (1995).
Richard, I. et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81, 27–40 (1995).
Zlotogora, J., Gieselmann, V. & Bach, G. Multiple mutations in a specific gene in a small geographic area: a common phenomenon? Am. J. Hum. Genet. 58, 241–243 (1996).
Lin, D., Black, S.M., Nagahama, Y. & Miller, W.L. Steroid 17α-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132, 2498–2506 (1993).
Ravichandran, K.G., Boddupalli, S.S., Hasemann, C.A., Peterson, J.A. & Deisenhofer, J. Crystal structure of hemoprotein domain of P450BM-3, a prototype for microsomal P450′s. Science 261, 731–736 (1993).
Hasemann, C.A., Kurumbail, R.G., Boddupalli, S.S., Peterson, J.A. & Deisenhofer, J. Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure 3, 41–62 (1995).
Lin, D., Zhang, L., Chiao, E. & Miller, W.L. Modeling and mutagenesis of the active site of human P450c17. Mol. Endocrinol. 8, 392–402 (1994).
Picado-Leonard, J. & Miller, W.L. Homologous sequences in steroidogenic enzymes, steroid receptors and a steroid binding protein suggest a consensus steroid-binding sequence. Mol. Endocrinol. 2, 1145–1150 (1988).
Kitamura, M., Buczko, E. & Dufau, M.L. Dissociation of hydroxylase and lyase activities by site-directed mutagenesis of the rat P450-17α. Mol. Endocrinol. 5, 1373–1380 (1991).
Zhang, L., Rodriguez, H., Ohno, S. & Miller, W.L. Serine phosphorylation of human P450c17 increases 17,20 lyase activity: implications for adrenarche and for the polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 92, 10619–10623 (1995).
Graham-Lorence, S., Amarneh, B., White, R.E., Peterson, J.A. & Simpson, E.R. A three-dimensional model of aromatase cytochrome P450. Protein Sci. 4, 1065–1080 (1995).
Graham-Lorence, S.E. & Peterson, J.A. Structural alignments of P450s and extrapolations to the unknown. Methods Enzymol. 272, 315–326 (1996).
Ferrin, T.E., Huang, C.C., Jarvis, L.E. & Langridge, R. The MIDAS display system. J. Mol. Graphics 6, 13–27 (1988).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Geller, D., Auchus, R., Mendonça, B. et al. The genetic and functional basis of isolated 17,20–lyase deficiency. Nat Genet 17, 201–205 (1997). https://doi.org/10.1038/ng1097-201
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1097-201
This article is cited by
-
Congenital adrenal hyperplasia, disorders of sex development, and infertility in patients with POR gene pathogenic variants: a systematic review of the literature
Journal of Endocrinological Investigation (2022)
-
Common variant rs11191548 near the CYP17A1 gene is associated with hypertension and the serum 25(OH) D levels in Han Chinese
Journal of Human Genetics (2018)
-
CYB5A polymorphism increases androgens and reduces risk of rheumatoid arthritis in women
Arthritis Research & Therapy (2015)