Abstract
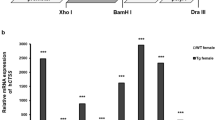
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disease that affects over one million people in the United States. SLE is characterized by the presence of anti-nuclear antibodies (ANA) directed against naked DNA and entire nucleosomes. It is thought that the resulting immune complexes accumulate in vessel walls, glomeruli and joints and cause a hypersensitivity reaction type III, which manifests as glomerulonephritis, arthritis and general vasculitis. The aetiology of SLE is unknown, but several studies suggest that increased liberation or disturbed clearance of nuclear DNA-protein complexes after cell death may initiate and propagate the disease1,2,3,4,5,6. Consequently, Dnase1, which is the major nuclease present in serum, urine and secreta, may be responsible for the removal of DNA from nuclear antigens at sites of high cell turnover and thus for the prevention of SLE (refs 7–11). To test this hypothesis, we have generated Dnase1-deficient mice by gene targeting. We report here that these animals show the classical symptoms of SLE, namely the presence of ANA, the deposition of immune complexes in glomeruli and full-blown glomerulonephritis in a Dnase1-dose-dependent manner. Moreover, in agreement with earlier reports10, we found Dnase1 activities in serum to be lower in SLE patients than in normal subjects. Our findings suggest that lack or reduction of Dnase1 is a critical factor in the initiation of human SLE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carroll, M.C. The lupus paradox. Nature Genet. 19, 3– 4 (1998).
Vanholder, R., De Keyser, F., Kips, J., Praet, M. & Naeyaert, J.M. The pathophysiology of lupus erythematosus. Eur. J. Dermatol. 1, 4–7 (1998).
Berden, J.H.M., Licht, R., Van Bruggen, M.C.J. & Tax, W.J.M. Role of nucleosomes for induction and glomerular binding of autoantibodies in lupus nephritis. Curr. Opin. Nephrol. Hypertens. 8, 299–306 (1999).
Rosen, A. & Casciola-Rosen, L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 6, 6– 12 (1999).
Eilat, D. & Naparstek, Y. Anti-DNA autoantibodies: a puzzle of autoimmune phenomena. Immunol. Today 20, 339–342 (1999).
Lachmann, P.J. An attempt to characterize the lupus erythematosus cell antigen. Immunology 4, 153–163 ( 1961).
Lacks, S.A. Deoxyribonuclease I in mammalian tissues. J. Biol. Chem. 256, 2644–2648 (1981).
Takeshita, H. et al. Mouse deoxyribonuclease I (Dnase I): biochemical and immunological characterization, cDNA structure and tissue distribution. Biochem. Mol. Biol. Int. 42, 65–75 (1997).
Peitsch, M.C., Polzar, B., Tschopp, J. & Mannherz, H.G. About the involvement of deoxyribonuclease I in apoptosis. Cell Death Differ. 1, 1–6 (1994).
Chitrabamrung, S., Rubin, R.L. & Tan, E.M. Serum deoxyribonuclease I and clinical activity in systemic lupus erythematosus. Rheumatol. Int. 1, 55–60 (1981).
Macanovic, M. & Lachmann, P.J. Measurement of deoxyribonuclease I (DNase) in the serum and urine of systemic lupus erythematosus (SLE)-prone NZB/NZW mice by a new radial enzyme diffusion assay. Clin. Exp. Immunol. 108, 220–226 ( 1997).
Peitsch, M.C., Irmler, M., French, L.E. & Tschopp, J. Genomic organisation and expression of mouse deoxyribonuclease I. Biochem. Biophys. Res. Commun. 207, 62–68 (1995).
Shimoda, M. et al. Anti-DNA IgA autoantibodies are spontaneously generated in mouse Peyer's patches. Immunology 95, 200 –207 (1998).
Polzar, B. et al. Distribution of deoxyribonuclease I in rat tissues and its correlation to cellular turnover and apoptosis (programmed cell death). Eur. J. Cell Biol. 64, 200–210 (1994).
Rumore, P.M. & Steinman, C.R. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J. Clin. Invest. 86, 69– 74 (1990).
Macanovic, M. et al. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin. Exp. Immunol. 106, 243–252 (1996).
Verthelyi, D., Dybdal, N., Elias, K.A. & Klinman, D.M. DNAse treatment does not improve the survival of lupus prone (NZB/NZW) F1 mice . Lupus 7, 223–230 (1998).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genet. 19, 56–59 (1998).
Bickerstaff, M.C.M. et al. Serum amyloid P component controls chromatin degradation and prevents anti-nuclear autoimmunity. Nature Med. 5, 694–697 (1999).
Davis, J.C. et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 8, 68– 76 (1999).
Nadano, D., Yasuda, T. & Kishi, K. Measurement of deoxyribonuclease 1 activity in human tissues and body fluids by a single radial enzyme-diffusion method. Clin. Chem. 39, 448–452 ( 1993)
Elkon, K.B., Parnassa, A.P. & Foster, C.L. Lupus autoantibodies target ribosomal P proteins. J. Exp. Med. 162, 459–471 (1985).
Moore, T.L., Weiss, T.D., Neucks, S.H., Baldassare, A.R. & Zuckner, J. Extractable nuclear antigens. Semin. Arthritis Rheum. 10, 309–318 (1981).
Polzar, B. & Mannherz, H.G. Nucleotide sequence of a full length cDNA clone encoding the deoxyribonuclease I from the rat parotid gland . Nucleic Acids Res. 18, 7151 (1990).
Acknowledgements
We thank B. Polzar for rat Dnase1 cDNA; P. Ferrier for the pPNT vector; E. Gau, E.-M. Konieczny, K. Klar, T. Klöckl and S. Wulf for technical assistance; and W. Zidek and M. Tepel for patient sera. This work was supported by a grant of the Fond der Chemischen Industrie to T.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Napirei, M., Karsunky, H., Zevnik, B. et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet 25, 177–181 (2000). https://doi.org/10.1038/76032
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76032
This article is cited by
-
Regulation of the nucleic acid-sensing Toll-like receptors
Nature Reviews Immunology (2022)
-
Renal Dnase1 expression is regulated by FGF23 but loss of Dnase1 does not alter renal phosphate handling
Scientific Reports (2021)
-
Evaluation of the functional effects of genetic variants‒missense and nonsense SNPs, indels and copy number variations‒in the gene encoding human deoxyribonuclease I potentially implicated in autoimmunity
Scientific Reports (2019)
-
DNA sensing by the cGAS–STING pathway in health and disease
Nature Reviews Genetics (2019)
-
Neutrophil extracellular traps-associated markers are elevated in patients with systemic lupus erythematosus
Rheumatology International (2019)